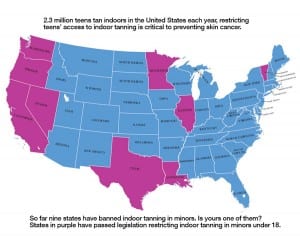
Sunlamp products and ultraviolet (UV) lamps are now moderate-risk (class II) devices. The new ruling also requires that sunlamp products carry a visible black-box warning on the device that states that the sunlamp product should not be used on persons under the age of 18 years. So far, nine states in the US have banned the use of indoor tanning beds for minors under 18. Is your state one of them? See infographic at right.
In addition, certain marketing materials for sunlamp products and UV lamps must include additional and specific warning statements and contraindications, ? including “Persons repeatedly exposed to UV radiation should be regularly evaluated for skin cancer.”
As part of today’s action, manufacturers will now have to submit a premarket notification (also called a “510(k)”) to the FDA – and obtain FDA clearance – prior to marketing these devices. Until now, sunlamp products were exempt from premarket review. Manufacturers also will now have to show that their products meet certain performance testing requirements and address certain product design characteristics.
The FDA’s final order for the reclassification of sunlamp products and UV lamps follows the recommendations from an advisory panel meeting in March 2010.


