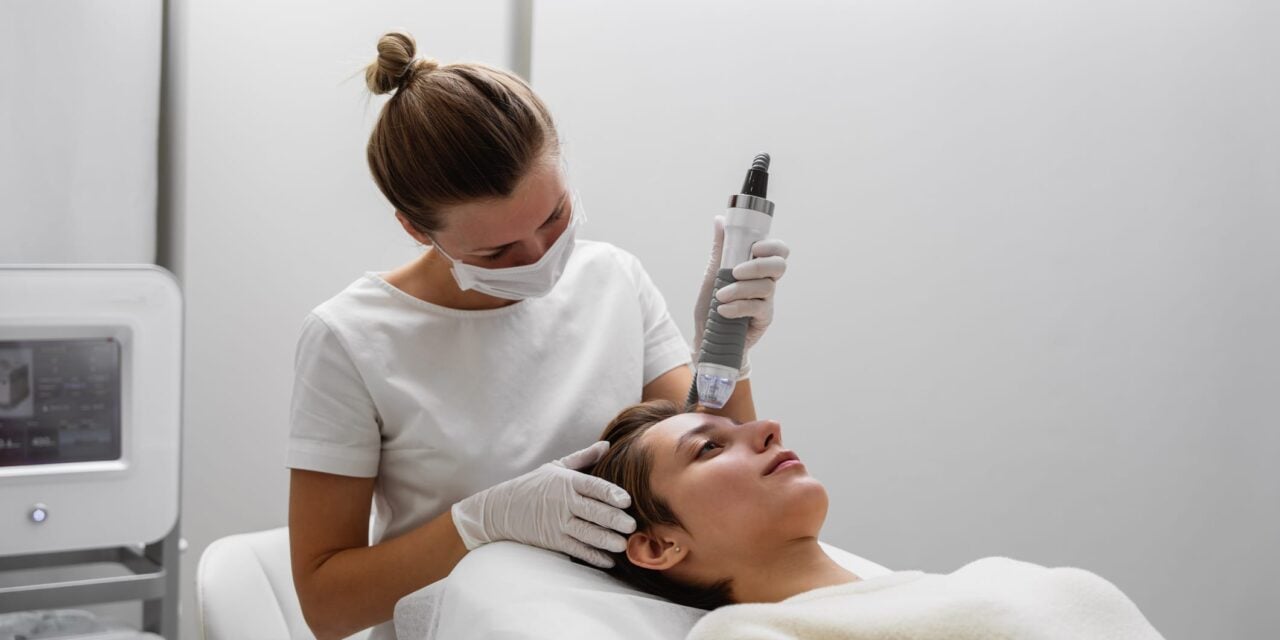
The agency has received reports of burns, scarring, fat loss, and nerve damage associated with the aesthetic devices and is urging providers to report adverse events.
The US Food and Drug Administration (FDA) has issued a safety communication alerting health care providers and patients to potential risks associated with radiofrequency (RF) microneedling devices.
The agency states it is aware of reports of serious complications when these devices are used for dermatologic or aesthetic procedures. The reported adverse events include burns, scarring, fat loss, disfigurement, and nerve damage, with some cases requiring surgical repair or other medical intervention.
RF microneedling devices, which are classified as Class II medical devices cleared through the 510(k) process, use an array of microneedles to deliver electrical energy to specific depths within and under the skin, creating localized heat to improve skin appearance.
The FDA’s evaluation of the issue is ongoing, and the agency is working with device manufacturers to identify potential mitigation strategies.
Recommendations for Health Care Providers
In its communication, the FDA outlined several recommendations for health care providers who use RF microneedling devices in their practice. The agency advises clinicians to:
- Be aware of the reports of serious complications, including burns, scarring, fat loss, disfigurement, and nerve damage, which may require intervention.
- Review and discuss the benefits and risks of all available dermatologic and aesthetic skin procedures with patients and their caregivers.
- Emphasize to patients that RF microneedling is a medical procedure, not a cosmetic treatment, and should not be performed with at-home devices.
- Report any problems or complications experienced by patients from procedures with RF microneedling devices to the FDA.
FDA Actions and Adverse Event Reporting
The FDA is actively monitoring reports of adverse events related to these devices to better understand the associated risks. The agency encourages health care personnel to report any issues through the MedWatch Voluntary Reporting Form.
The FDA will continue to inform the public if significant new information becomes available.