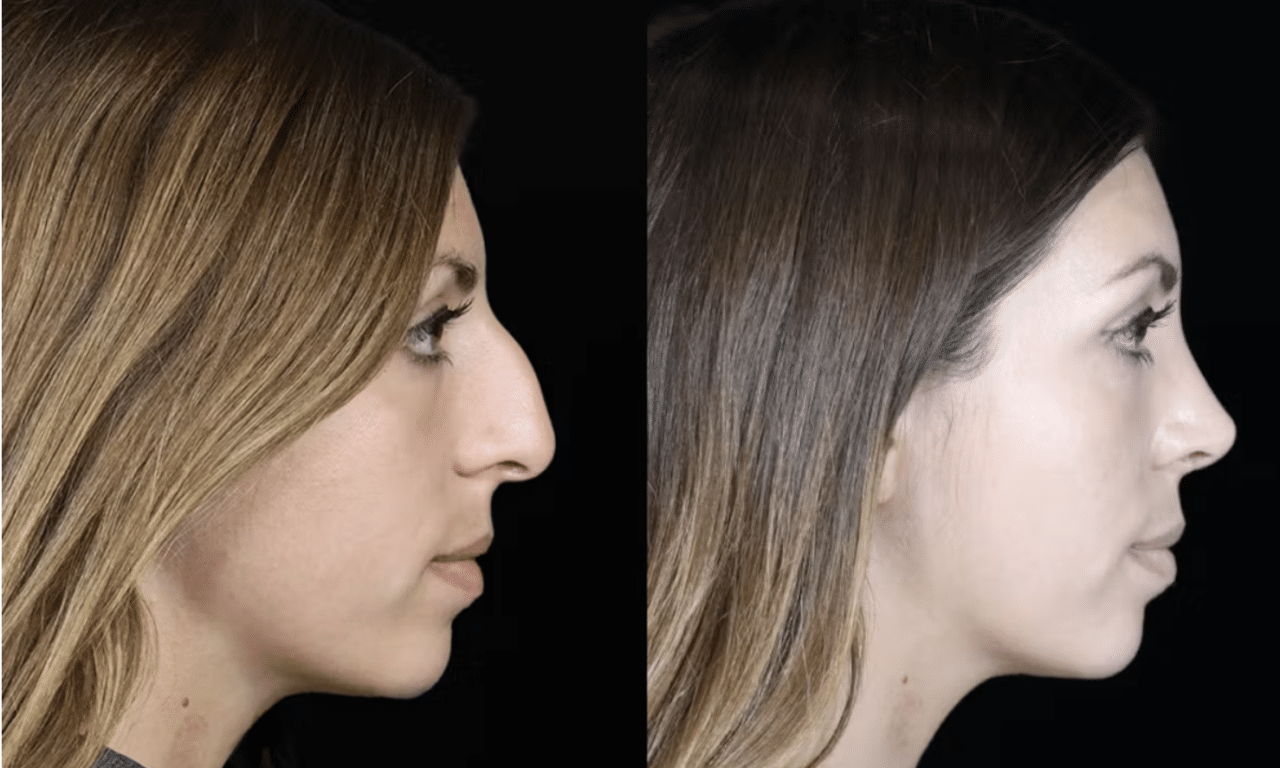
Ellacor and the Rise of Micro-Coring: Insights from the Clinic and the Company
Plastic surgeon Gaurav Bharti, MD, FACS, and Cytrellis Biosystems CEO Denise Dajles, D.Eng, share clinical and strategic insights into how ellacor is changing the way skin laxity and facial wrinkles are treated without surgery or thermal energy.