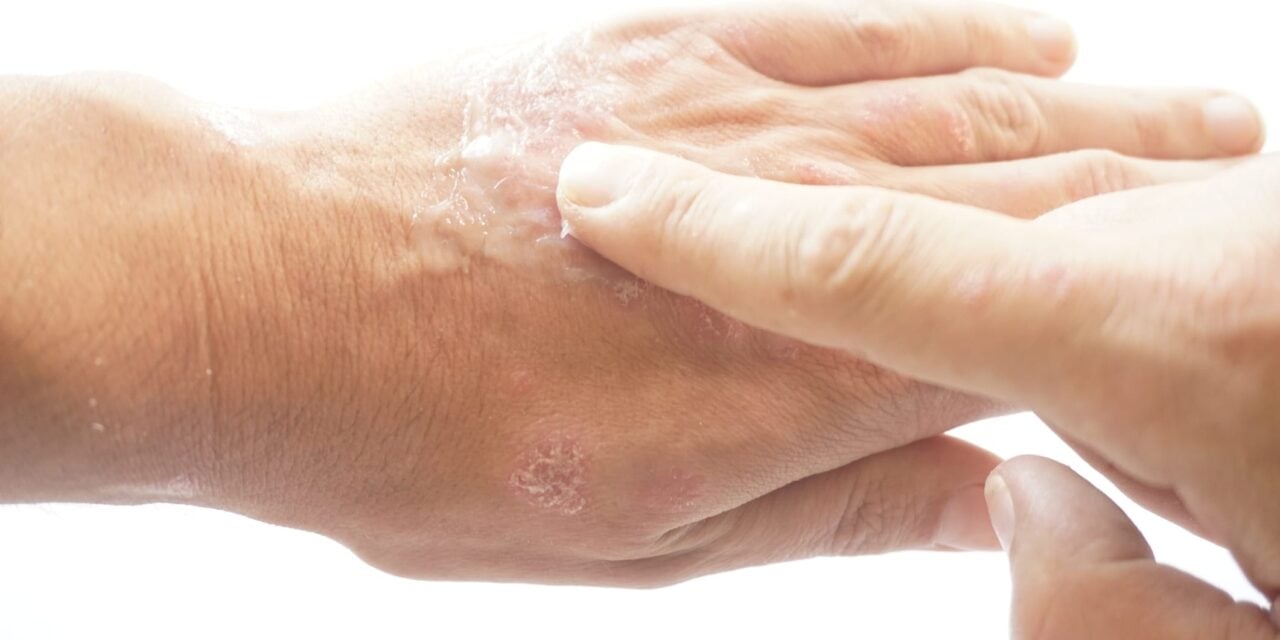
The American Academy of Dermatology issued four strong evidence-based recommendations for the treatment of atopic dermatitis in adults in its 2025 focused update.
The American Academy of Dermatology (AAD) has published a 2025 focused update to its clinical guidelines for managing atopic dermatitis (AD) in adults, issuing strong evidence-based recommendations for four therapies: tapinarof (Vtama) cream, roflumilast (Zoryve) cream, lebrikizumab (Ebglyss), and nemolizumab (Nemluvio) with concomitant topical therapy.
According to the AAD, several novel therapies have emerged since the publication of previous guidelines in 2023 and 2024. This update brings those guidelines up to date. A multidisciplinary workgroup conducted a systematic review and applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of evidence and develop recommendations.
Tapinarof (VTAMA) cream, 1%, a steroid-free topical treatment, was among the therapies granted a strong recommendation. Organon, the manufacturer of Vtama, notes that the recommendation was supported by findings from the ADORING 1 and ADORING 2 eight-week clinical trials. The company also pointed to results from its phase 3 ADORING 3 48-week, open-label, long-term extension study, which found that after treatment success, patients experienced a treatment-free interval lasting on average 80 days.
All four therapies received FDA approvals in 2024 for various atopic dermatitis indications. In July 2024, the FDA approved Arcutis’ Zoryve (roflumilast) cream 0.15% for AD in adults and children as young as 6. Eli Lilly’s Ebglyss (lebrikizumab) received FDA approval in September 2024 for moderate-to-severe AD in adults and children 12 years and older who are not adequately controlled with topical therapies. Vtama was approved in December 2024 for the topical treatment of AD in adults and pediatric patients 2 years of age and older. Most recently, in December 2024, Galderma’s Nemluvio (nemolizumab) was approved for moderate-to-severe AD in patients 12 years and older, in combination with topical corticosteroids and/or calcineurin inhibitors when the disease is not adequately controlled with topical prescription therapies.
The AAD’s 2025 focused update is part of a broader series of updates to its 2014 guidelines, aiming to ensure dermatologists have access to the most current evidence for treating adult AD. The update incorporates recently FDA-approved topical and systemic therapies into existing guidance.
ID 158338279 | Patient © Djvo16 | Dreamstime.com