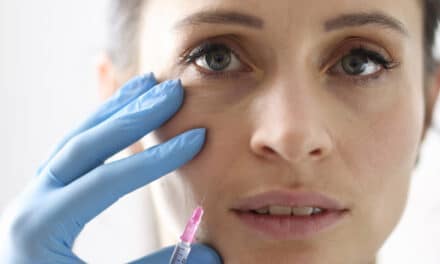
The agency has accepted the resubmission seeking approval of the neuromodulator for the temporary improvement of moderate-to-severe glabellar lines and crow’s feet in adults.
The US Food and Drug Administration (FDA) has accepted Galderma’s Biologics License Application (BLA) resubmission for RelabotulinumtoxinA for the temporary improvement of moderate-to-severe glabellar lines (frown lines) and lateral canthal lines (crow’s feet) in adults.
Galderma says in a press release that it has worked with the US FDA to implement adjustments to its manufacturing process.
“We pioneered the development of RelabotulinumtoxinA to address the growing demand for faster-acting and longer-lasting anti-wrinkle solutions. We’re excited about the potential to bring this innovative neuromodulator to the US, offering advanced performance and ease of use and building on our portfolio of neuromodulators that meets the full spectrum of injector and patient needs,” says Baldo Scassellati Sforzolini, MD, PhD, global head of research and development at Galderma, in a release.
The filing is based on data from the large-scale READY (REelabotulinumtoxin Aesthetic Development StudY) clinical trial program, which is composed of four phase III trials, enrolling more than 1,900 participants. Results demonstrated that RelabotulinumtoxinA delivered a fast onset of action as early as day 1 and sustained results for six months for both frown lines and crow’s feet.
RelabotulinumtoxinA (Relfydess) has been approved in over 20 markets for the treatment of frown lines and crow’s feet, including in the European Union, the United Kingdom, Asia, and Australia. Regulatory applications are continuing to be submitted and assessed by additional authorities globally.
ID 418119372 © Anatolii Rabizo | Dreamstime.com