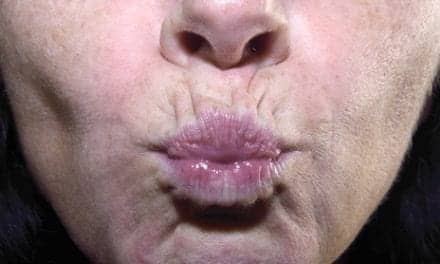
The US Food and Drug Administration (FDA) has given its thumbs-up to the dermal fillers Restylane Refyne and Restylane Defyne for the treatment of nasolabial folds (aka “laugh lines”), as well as to Eucrisa (crisaborole) ointment to treat eczema.
According to a media release from Fort Worth, Tex-based Galderma, Restylane Refyne was approved for the treatment of moderate to severe facial wrinkles and folds, and Restylane Defyne was approved for the treatment of moderate to severe, deep facial wrinkles and folds, in patients over the age of 21. Both are manufactured with XpresHAn Technology.
A separate media release from the FDA announces its approval for Eucrisa (crisaborole) ointment, manufactured by Pfizer Inc to treat mild to moderate eczema (atopic dermatitis) in patients 2 years of age or older.
Eucrisa, applied topically twice daily, is a phosphodiesterase 4 (PDE-4) inhibitor, although its specific mechanism of action in atopic dermatitis is not known, the release adds.
For more information, visit the US Food and Drug Administration.
[Source(s): US Food and Drug Administration, PR Newswire]