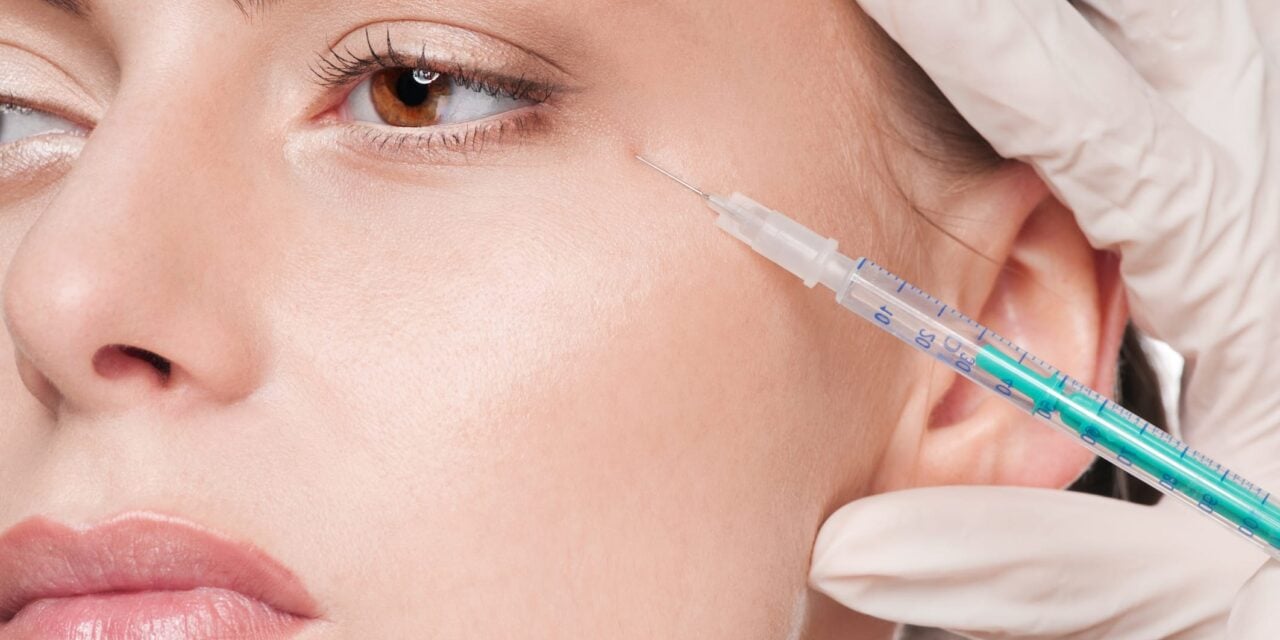
The FDA issued 18 warning letters to online sellers of unapproved and misbranded botulinum toxin products linked to serious safety risks.
The US Food and Drug Administration (FDA) issued 18 warning letters to owners of websites illegally marketing unapproved and misbranded botulinum toxin products. The agency is aware of adverse events associated with unapproved and misbranded botulinum toxin products, including botulism symptoms.
“Unapproved and misbranded Botox products carry serious health risks. Today we’re taking action to protect American consumers and prevent online entities from selling these dangerous products,” says FDA commissioner Marty Makary, MD, MPH, in a release.
FDA-approved botulinum toxin products carry a boxed warning, the agency’s most serious warning, indicating the drug carries a significant risk of serious or life-threatening side effects. The boxed warning indicates the product may cause symptoms of botulism.
Patients should ensure they only receive these products from a provider who is licensed and trained to administer such injections, according to a release from the FDA. Additionally, patients should only receive these injections if the product is obtained from an authorized source. Products purchased from unauthorized sources may be unapproved, misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective, and/or unsafe.
The FDA says to seek immediate medical care for symptoms of botulism, including trouble swallowing or breathing, after receiving a botulinum toxin product injection.
The FDA issued warning letters to:
- acecosm.com
- aesthetic-essentials.com
- celestapro.com
- cosmenic.net
- cosmo-korea.com
- derma-solution.com
- dermaxshop.com
- ellepharm.com
- estaderma.com
- filleroutlet.com
- glamderma.com
- glowface.store
- glownestbeauty.com
- koreafillerexperts.com
- koreanfillers.com
- maypharm.net
- meamoshop.com
- mjsmedicals.com
Health care professionals and consumers can report adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.