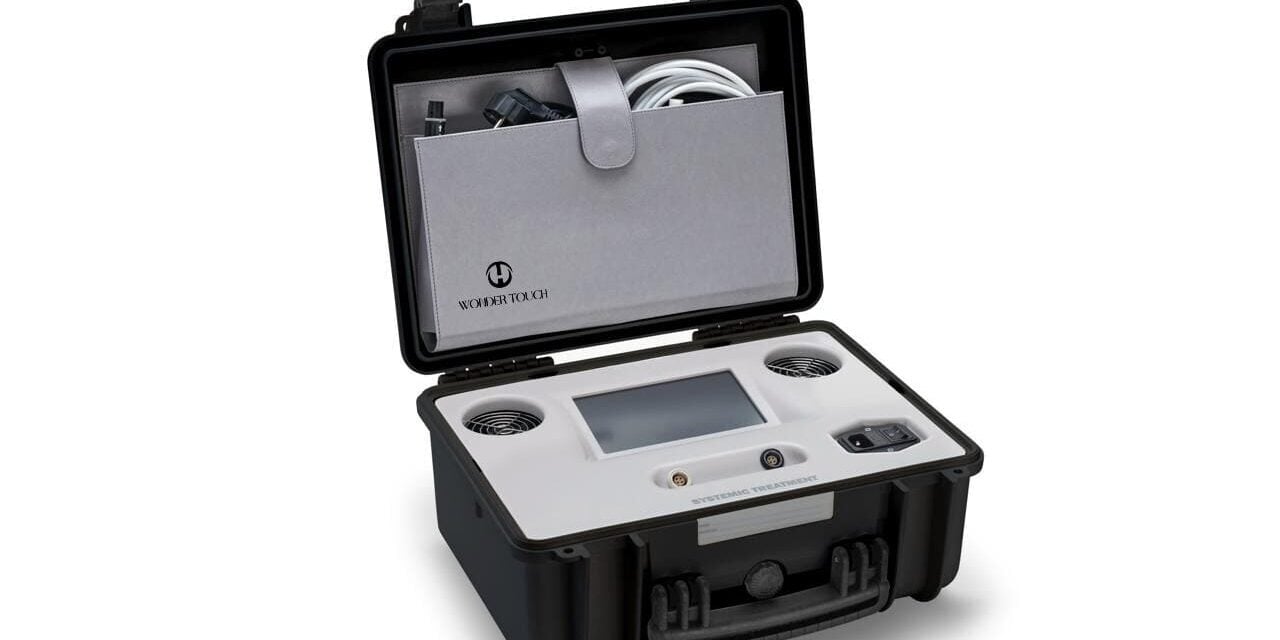
The FDA-cleared device delivers heat through a hands-on, energy-based facial massage technique for skin tightening, textural improvements, and localized fat reduction.
XOD (Aesthetics On Demand) announced the US launch of Wonder Touch, a US Food and Drug Administration-approved, needle-free portable radiofrequency device that delivers heat through a hands-on, energy-based facial massage technique. Wonder Touch is designed to offer improved patient results for skin tightening, textural improvements, and localized fat reduction.
Portable and compact, Wonder Touch was designed with mobility in mind, allowing providers to expand to satellite locations, pop-up events, and VIP concierge offerings.
“We designed and developed Wonder Touch to meet the demand for treatments that are effective, comfortable, and flexible enough to fit any business model,” says Lee Haimoff, CEO and co-founder of XOD.
Clinical Excellence Leadership Team Expands
Additionally, XOD announced the appointment of Kelly Hermans, CRNA, to its clinical excellence leadership team. Hermans is an injector, educator, and entrepreneur who founded the Beautiphi Aesthetic Boutique and Beautiphi Academy. Hermans brings decades of experience helping aesthetic providers implement advanced treatments and build scalable, sustainable practices. She is the first aesthetic provider in Michigan to integrate XOD’s Breeze and Wonder Touch devices into her practice.
“Wonder Touch is the perfect example of innovative technology and is unlike anything else on the market,” says Hermans in a release. “It delivers results with the ease and elegance of a massage, not needles—and that is a game-changer. I’m excited to see how this energy-based, massage device and the Breeze, which uses the power of barophoresis and microdroplets, will elevate the results and patient experience of my injectables, laser-prepping, and post-laser treatments.”
Photo caption: Wonder Touch
Photo credit: XOD