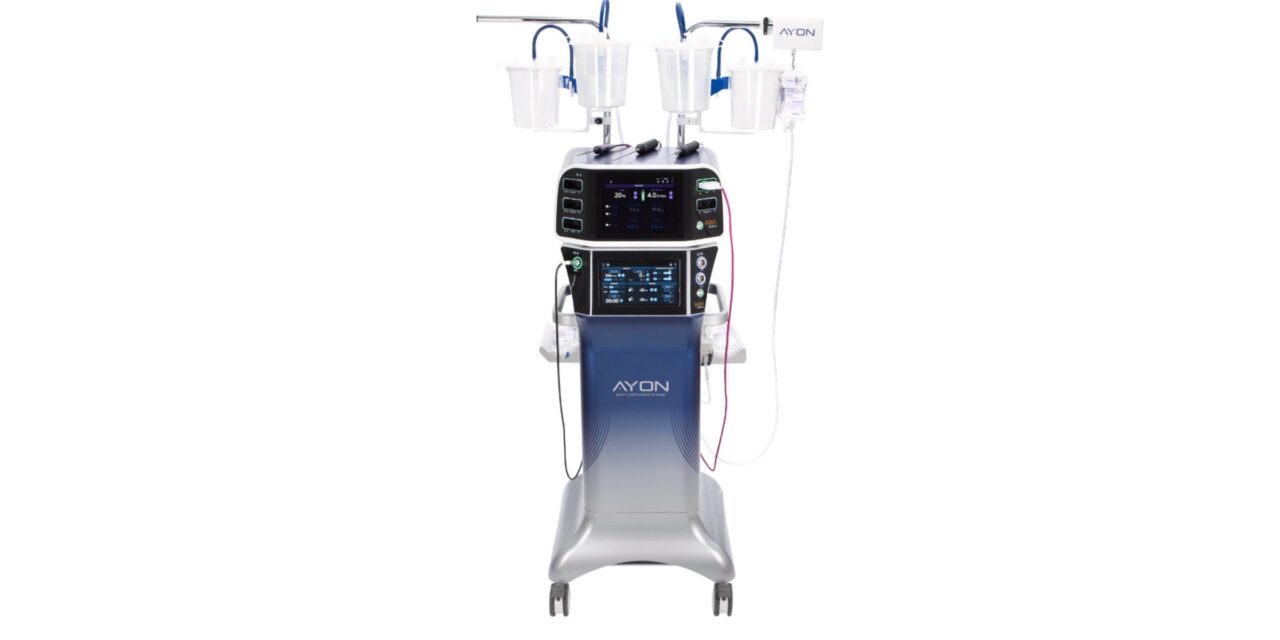
The potential clearance would add power liposuction to the all-in-one platform, creating a single device for multiple contouring procedures.
Apyx Medical Corp has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) to expand the label for its Ayon Body Contouring System to include power liposuction.
If cleared, the addition would make Ayon the first fully integrated body contouring system, according to the company. The platform already combines fat removal, closed-loop contouring, tissue contraction, and electrosurgical capabilities. The proposed power liposuction function uses a reciprocating liposuction cannula intended to reduce the manual effort required by surgeons during fat removal.
“Upon receiving FDA clearance for power liposuction, we will be able to activate the new functionality in the Ayon systems already installed at surgical centers across the US through the ongoing commercial launch,” says Charlie Goodwin, president and CEO, in a release. “The feedback we have received from the early adopters of Ayon for the currently cleared aesthetic procedures is overwhelmingly positive, and we are excited to have recently expanded our commercial rollout to aesthetic surgeons across the US.”
The company began the nationwide commercial launch of the Ayon system in September 2025. Apyx Medical is also hosting a virtual key opinion leader event on Oct 14, 2025, featuring Paul Vanek, Jr, MD, FACS, founder, president, and CEO of Mentor Plastic Surgery & MedSpa, to discuss the system’s commercial launch.
Photo caption: Ayon Body Contouring System
File photo/Apyx Medical Corp