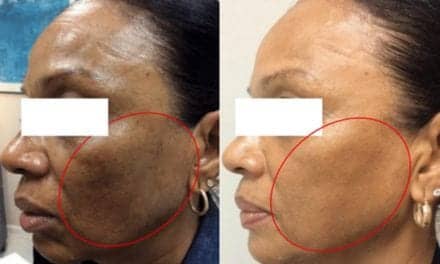
Five-year data show that Bellafill® is safe and effective for the treatment of nasolabial folds—with a high level of patient satisfaction, Suneva Medical reports.
Five-year data show that Bellafill® is safe and effective for the treatment of nasolabial folds—with a high level of patient satisfaction, Suneva Medical reports.
The 5-year post-approval study aimed to determine Bellafill’s long-term safety. Effectiveness was a secondary objective. In it, 1,008 patients were enrolled across 23 US centers. At 5 years, there was an 87% retention rate, with the majority of patients (83%) rating a “satisfied” to “very satisfied” outcome.
“Longer acting does matter, and the fact that this product has a permanent component is mission critical.” —Frank Fazio, vice president, corporate development at Suneva Medical Inc
No treatment-related serious adverse events or unanticipated adverse events were noted. The incidence of treatment-related adverse events (TRAEs) in patients was 11.7%. The majority of these events were mild in severity and resolved by the conclusion of the study. The most commonly reported TRAEs were lumpiness at the injection site (~4.5%) and redness (1.8%). The incidence of granulomas was infrequent (1.7%).
 “The strong supporting evidence of Bellafill’s safety profile supplies data that allow physicians to use the filler with confidence, dispelling misconceptions about the product’s long-term safety profile including the very low rate of granulomas, which can occur with any dermal filler,” says Steven Cohen, MD, and clinical professor at the University of California, San Diego, in a news release.
“The strong supporting evidence of Bellafill’s safety profile supplies data that allow physicians to use the filler with confidence, dispelling misconceptions about the product’s long-term safety profile including the very low rate of granulomas, which can occur with any dermal filler,” says Steven Cohen, MD, and clinical professor at the University of California, San Diego, in a news release.
 “Longer acting does matter, and the fact that this product has a permanent component is mission critical,” says Frank Fazio, vice president, corporate development at Suneva Medical Inc. “Many patients today have dermal filler fatigue, and are ready to move on to longer-lasting fillers.”
“Longer acting does matter, and the fact that this product has a permanent component is mission critical,” says Frank Fazio, vice president, corporate development at Suneva Medical Inc. “Many patients today have dermal filler fatigue, and are ready to move on to longer-lasting fillers.”
The new study results can’t be extrapolated to include Bellafill’s acne scar indication, Fazio adds.
Suneva Medical Inc rebranded ArteFill® as Bellafill in late 2014. The company opted for the name change because Bellafill better embodies the outcomes associated with the filler. Check out the new sizzle reel on the rebrand here.