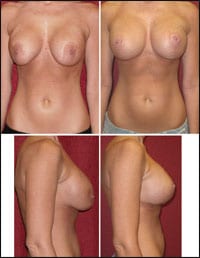
BTL Aesthetics launches the small applicator and presents eight studies on EMSCULPT at the 39th Annual Conference of the American Society for Laser Medicine & Surgery (ASLMS), March 27 to 31 in Denver.
Up until now, there were only FDA-cleared protocols for the abdominals and buttocks. With the addition of the small applicator, EMSCULPT is now cleared for strengthening, firming, and toning of arms and thighs, further expanding the device’s versatility for physicians, the company notes in a media release.
“We at BTL are always researching new innovations and raising the bar in aesthetics in order to improve patient satisfaction and outcomes,” shares John Ferris, vice president of US Marketing at BTL, in the release.
“We’re looking forward to sharing our latest findings with the esteemed attendees of ASLMS and being the driving force behind the ongoing evolution of the category,” he adds.
The presentations highlight new clinical findings demonstrating long-term EMSCULPT results with 6-month and 1-year data. Research is also being unveiled showcasing the results for the new applications.
Brand presentations during the ASLMS conference include:
Title: Ultrasonography Evaluation of Changes in Subcutaneous Abdominal Fat Thickness Following HIFEM Treatments: Results of 6-Month Follow-Up
Date: March 29
Time: 4:08 – 4:13 PM
Product: EMSCULPT
Presenter: Dr. Bruce E. Katz
Category: Clinical Applications – Multispecialty
Title: Body Contouring Device
Date: March 29
Time: 5:45 – 5:48 PM
Product: EMSCULPT
Presenter: Dr. Bruce E. Katz
Category: Tech Connect
Title: Combining Cryolipolysis and HIFEM to Optimize Body Contouring and Other Latest Developments
Date: March 30
Time: 7:16 – 7:28 AM
Product: EMSCULPT
Presenter: Dr. Suzanne Kilmer
Category: Full Spectrum of Body Contouring
Title: Non-Invasive Induction of Muscle Fiber Hypertrophy and Hyperplasia: Effects of High-Intensity Focused Electromagnetic (HIFEM) Field Evaluated in an In Vivo Porcine Model
Date: March 30
Time: 1:34 – 1:41 PM
Product: EMSCULPT
Presenter: Dr. Diane Duncan
Category: Basic Science and Transitional Research
Title: Biochemical Perspective of Fat Physiology After Application of HIFEM Field Technology: Additional Investigation of Fat Disruption Effects in A Porcine Study
Date: March 30
Time: 3:31 – 3:38 PM
Product: EMSCULPT
Presenter: Dr. Yael Halaas
Category: Basic Science and Transitional Research
Title: MRI Evaluation of Changes in Gluteal Muscles Following Treatments with the High-Intensity Focused Electromagnetic (HIFEM) Technology
Date: March 30
Time: 4:33 – 4:38 PM
Product: EMSCULPT
Presenter: Dr. Melanie D. Palm
Category: Clinical Applications – Cutaneous
Title: Long-Term Follow-Up on Patients with HIFEM-Induced Abdominal Tissue Changes: MRI and CT Assisted Quantification of Muscle Growth and Fat Reduction
Date: March 30
Time: 4:39 – 4:44 PM
Product: EMSCULPT
Presenter: Dr. David Kent
Category: Clinical Applications – Cutaneous
Title: Histological Examination of Skin Tissue in Porcine Animal Model After Simultaneous Application of Monopolar Radiofrequency and Targeted Pressure Energy
Date: March 31
Time: 8:25 – 8:32 AM
Product: BTL Aesthetics
Presenter: Dr. Brian Kinney
Category: Basic Science and Translational Research
[Source(s): BTL Aesthetics, PR Newswire]