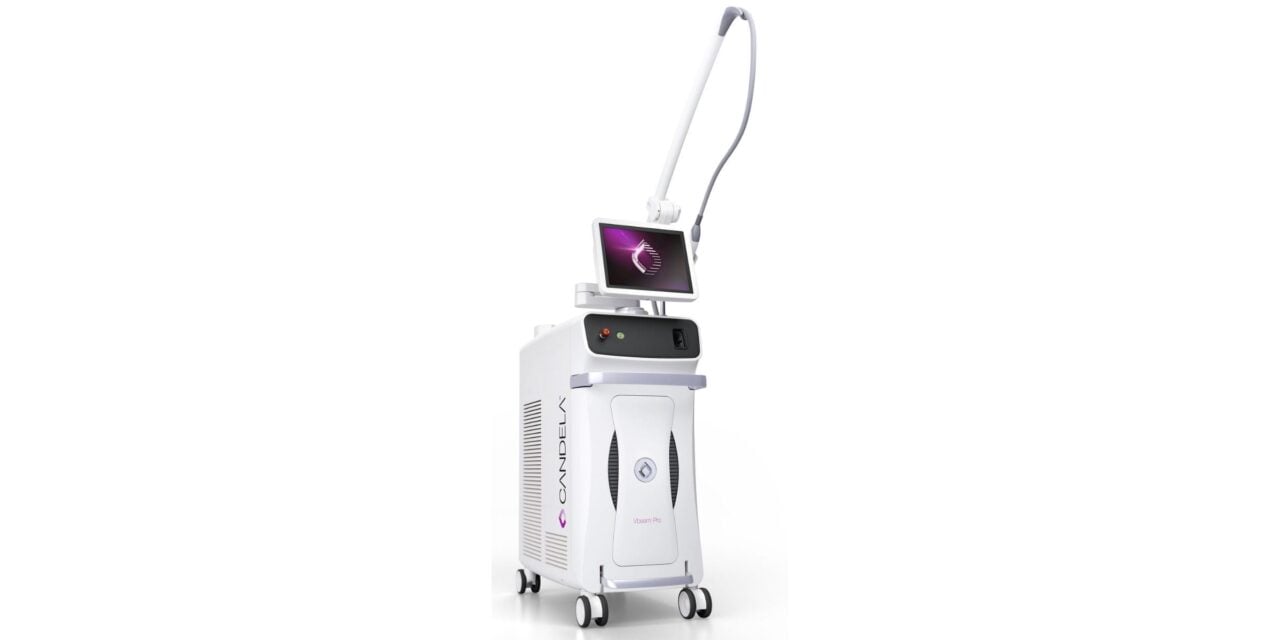
The platform, cleared for pediatric and adult use, combines a 595 nm pulsed dye laser with a 1064 nm Nd:YAG wavelength and features improved treatment speed, precision, and patient comfort.
Candela Corp announced the launch of the Vbeam Pro platform for the treatment of vascular and dermatologic conditions at the 2025 Annual Meeting of the American Society for Laser Medicine and Surgery.
The Vbeam Pro device, US Food and Drug Administration-cleared for use in adult and pediatric patients, combines a 595 nm pulsed dye laser with a 1064 nm Nd:YAG wavelength. It’s designed to treat a broad spectrum of skin conditions, including rosacea, port-wine stains, acne, leg veins, spider veins, scars, benign pigmented lesions, wrinkles, warts, stretch marks, and photoaging.
“The Vbeam Pro system is a transformative evolution in pulsed dye laser technology,” says Roy Geronemus, MD, clinical professor in the department of dermatology at NYU Grossman School of Medicine and medical director of the Vascular Birthmarks Foundation, in a release. “The clinical results are remarkably consistent, and patients are experiencing enhanced outcomes compared to earlier-generation systems,” he adds.
With its dual wavelengths and 30% tighter energy delivery control, the Vbeam Pro laser offers a new level of efficacy and efficiency for clinicians treating patients across all Fitzpatrick skin types (I–VI). The platform also features optimized dye life and an enhanced user interface.
Backed by rigorous clinical research, the Vbeam Pro laser has demonstrated:
- 20% improved clearance of treated lesions¹
- 50% faster treatment times²
- 20% reduction in reported patient discomfort³
“Reinforcing Candela’s commitment to scientific rigor, the Vbeam Pro system underwent extensive clinical evaluation at leading dermatology practices across the United States,” says Konika Patel Schallen, MD, senior vice president of clinical operations and medical director at Candela, in a release. “We put the platform through stringent testing, using it in busy clinical environments on a wide variety of skin types and conditions. We are excited about the feedback from our dermatology users on enhancing patient outcomes and treatment efficiency.”
Photo caption: Vbeam Pro Device
Photo credit: Candela Corp
References
- Compared to Vbeam Perfecta; Vbeam 595 nm output Accuracy and Precision data on file
- Supported by early LCR participant treatment feedback
- Data from LCR sites relative to Perfecta