 |
Rather than use a rectus free flap to obliterate dead space and isolate the nasopharynx from the intracranial cavity, a Beverly Hills, Calif, facial plastic and reconstructive surgeon participating last year in the resection of a pernicious sinus tumor instead chose a free flap harvested from the patient’s forearm to cover the eye, skull base, infratemporal fossa, and other involved facial structures of a sinus cancer patient.
Babak Azizzadeh, MD, preferred the radial forearm free flap because its healing properties were superior to local and regional flaps—this was important, considering that the patient’s ability to mend had been degraded by his earlier exposure to fairly high-dose radiation while unsuccessfully undergoing palliative treatment. Furthermore, the radial forearm free flap offered two additional and no less crucial advantages. First, it would permit better postoperative visualization should the tumor recur. Second, it would most easily enable the patient to receive a midface prosthesis to achieve a truly good aesthetic result.
Azizzadeh says, “I like using radial forearm free flaps for reconstruction when the complexities of a case are such that local and pedicled flaps prove infeasible.”
Surgery the Only Choice
And complex this case undeniably was. It centered around a 62-year-old Southern California male who had sought medical help for a complaint of persistent numbness in his left cheek and tearing of his left eye. Symptoms were present for about 2 months before he was seen by Madison F. Richardson, MD, a nationally known head-and-neck surgeon.
“After customary conservative treatments for infection were found to have no effect, I suspected a tumor, so I ordered a CT scan, which demonstrated that the left sinus was indeed filled with a mass,” says Richardson, whose office is also in Beverly Hills. “Subsequent biopsy demonstrated it to be a high-grade cancer. Maxillary sinus cancer affects approximately one in 200,000 people. Among the most famous ever to develop it was President Ulysses S. Grant.”
Richardson referred his patient for radiation therapy and chemotherapy. Initial response was good, but the cancer soon recurred, Richardson reports. “What I found on examining the patient at this stage was an eye in a proptotic state,” he says. “Later, it was determined that the cancer had started to grow between the skin and bone of his eye socket—at the lower outside of his eyeball was a patch of necrotic tumor measuring approximately 3 inches.” (See Figures 1 and 2, page 68.)
Now, the patient’s only chance for survival was surgery.
Team Approach Mandated
The type of surgery Richardson contemplated was daunting enough to convince him that a team approach would prove the wisest course. Accordingly, he summoned cancer-skilled specialists from six key fields to help conduct the operation, expected to last about 15 hours.
“Fortunately, here in our Los Angeles area, we have talented physicians to draw upon when help is needed,” Richardson says. “At the very least, a case such as this requires the participation of a reconstructive plastic surgeon and a dental prosthodontist because, when you remove the maxilla, which we expected to do, you take out the masticatory apparatus along with the facial configuration.
“But you also must have the involvement of an ophthalmologist and a neurosurgeon—in 75% of these cases, the cancer will have spread up into the eye and on toward the brain. And, of course, it goes without saying that you must have as well a top anesthesiologist and an excellent pathologist.”
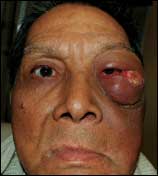 |
| Figure 1. Before surgery, the 62-year-old male patient had significant proptosis and a tumor on the left zygomatic and orbital region. |
After Richardson assembled his team, they met to plan the surgery and postoperative care. “Our goals of surgery were to remove all of the tumor, establish maximum function for speech and swallowing, and restore acceptable cosmetic appearance,” Richardson says. “As we developed our plan, we kept in mind that we likely were going to lose the entire palate and be forced to work up toward the brain. We also came up with contingency plans for every aspect of the surgery.”
Considerable Prep Work
The surgery took place at Cedars-Sinai Medical Center in Los Angeles. The first 90 minutes were devoted to prepping the patient—inserting catheters and central lines to enable easier and more accurate monitoring of vital signs and fluid output, as well as permit more precise administration of anesthesia. “We also had to suture shut the eyelid of the noninvolved right eye as a precaution against corneal injury,” Richardson says. “And we had to use a nasogastric tube so that he could be fed during the first few postoperative days when he would be unable to swallow.”
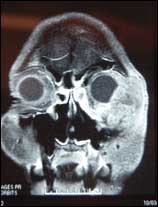 |
| Figure 2. A preoperative x-ray scan shows the extent of the tumor. |
With the prepping complete, the team moved on to marking the incision site. The surgeons—with Richardson acting as team leader—concurred that the inked marks should extend beyond the visible and tactile observable area of the cancer by an additional 3¼4 inch. “We knew the cancer was aggressive and extended deep into the bone,” Richardson says. “It turned out, however, that it had gone slightly deeper than we were able to appreciate from our initial examinations.”
Richardson discloses that no computer-assisted, image-guided tools were used to determine the incisions or navigate the path of the cuts. “In my own practice, I use those tools on a daily basis for treating inflammatory infectious sinus disease,” he says. “But that’s in order to avoid critical structures. In this particular case, we needed to cut through the critical structures in order to extirpate the cancer from every possible place where it could be hiding.
“For example, we knew that at one point in the surgery we would be encountering the apex of the orbit where not only the optic nerve comes through but also some very large vessels. Again, the only reason to have used a navigator tool would be if we wanted to avoid those large vessels, which we did not.”
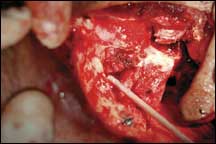 |
| Figure 3. The extent of the defect after orbital exenteration. |
For the most part, Richardson performed his initial and subsequent incisions with the aid of conventional instrumentation, keeping within reach and at the ready several versions of each tool. “I try to avoid becoming dependent on any one instrument because, if something goes wrong with it or it’s not available, then you can become very distracted and easily lose your place in the procedure while you hunt for a replacement,” he says. “So I try to have two or three instrument options for every part of the procedure. That way, if one instrument is not working, I’ve got immediate backup that I can go to without breaking my stride.”
Two of Richardson’s choices of tools were interesting for the reason that neither can be considered state-of-the-art. One was a familiar suction-cautery tool, which allowed him to clear blood from a field, locate the source of the bleeding, and then immediately cauterize it without having to pick up a second tool.
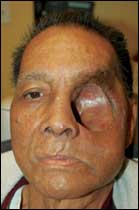 |
| Figure 4. The defect as it appeared 1 month after reconstruction with a radial forearm free flap. |
The other noteworthy instrument was a Gigli saw, first introduced in the 1940s—basically a wire saw, about 12 inches long, with a loop on each end to receive a handle. “You put it in the back of the hard palate and draw it from side to side to cut the tissues,” Richardson says. “It’s actually a lot faster than the power-driven tools available today. I think there is much merit still to the time-tested ways, and that’s why I prefer them.”
Establishing Margins
Richardson led off with an incision near the patient’s left eye. He cut above and around the eye, then brought the incision down to the middle of the lip and continued cutting toward the bone under the nose, over the maxillary sinus and up into the eye cavity.
The eyeball itself had to be lifted up from its socket in order to extirpate the cancer in that area. “Our objective was to bring the eye out laterally, still attached to the maxilla,” Richardson recounts.
To remove the eye, Richardson started by taking off the zygoma and disarticulating it there at the fissure. Next, he worked down to the cheekbone, cut across the zygoma, and proceeded back over into the mouth, there cutting the palate to split the face in half at its midline. “My earlier cuts had already freed up the eye laterally, superiorly, and partially posteriorly, but this freed it up medially as well,” he explains.
It happens that the eye socket is situated in one of the most blood-enriched parts of the body—there are large blood vessels everywhere. That is beneficial for promoting wound healing, but by the same token the messiness during surgery is significant enough to greatly obscure visualization of the area (Figure 3).
“A technique I used to sufficiently free the eye so that it could be lifted out of the socket did not involve visualization,” Richardson says. “All I did was carefully position the eye in my hand and then gave it a slight twist. Doing so enabled me to feel where it breaks along the pterygoid plates behind the maxillary sinus. I did this to make sure our incisions did not penetrate deep into the base of the skull.
“That was critical because that last cut has to be done almost entirely blind. You can’t really see, so you have to feel your way with your fingers, keeping your scissors or knife right next to your finger in order to free up those tissues, while your other fingers are working to protect the critical structures you don’t want to cut. There are a lot of muscles that close the jaw—the masticator, the temporalis, and the pterygoid muscles. They all have to be cut through, and they all have large veins associated with them.”
No Free Spaces
Richardson emphasizes that, as contrasted with other areas of the body, there are no free spaces in the head, neck, and face. “You will encounter, at every centimeter along the way, something that is involved in performing critical functions for something else,” he says. “The eye socket, in particular, has very limited space for you to work in, and so it becomes a bit tricky trying to get clamps in place when that space is filled with tumor.”
After this, the maxilla itself was lifted out. Then it was time to check for margins. Richardson says, “We went around and took tissue samples from the palate and deep in the eye cavity; these were analyzed by the pathologist.”
However, margins were not easily established. “The samples kept coming back showing that tumor was present up on the bone to the outer part of the eye going up toward the brain,” Richardson reveals. “So I would shave it down with a burr to the point where we started getting into the brain cavity. I got right down to the dura.
“The neurosurgeon on our team then made decisions as to how much of the dura should be taken as part of the margin. His concern was that, if we took too much without repairing it afterward, it would set up a situation where spinal fluid could leak into the eye and create the potential for meningitis.”
Reconstruction Begins
Once the tumor was fully resected, it became plastic surgeon Azizzadeh’s turn at bat. His first task was to determine which of three possible approaches he would take to cover the defect.
“With head and neck plastic reconstructive surgery, you never really know what you’re going to encounter until the tumor is completely removed,” Azizzadeh says. “In this very complex case, we did not know at the start of surgery how much tumor burden the patient had, how much of the tissue would be gone, what areas would be gone, and what the defect would be afterward. Because of those unknowns, we really had to come to the table prepared for any eventuality. If ever there was a case where failure to prepare amounted to preparing for failure, this was it.”
The first of the three potential plans Azizzadeh had formulated called for use of a skin graft if it turned out that only a small portion of the face and orbit were removed. “This would have been the most straightforward reconstructive effort,” he says.
The second approach under consideration envisioned obliterating the entire eye and midface with a rectus free flap. “The idea would have been to put in a substantial amount of muscle-, fat-, and skin-laden tissue, similar to what one might do in reconstructing a breast with a TRAM flap,” he says. “But this would not have yielded a particularly pleasing aesthetic result. It’s something you use when you have a very sizable tumor defect with a lot of intracranial or brain issues involved and you want an in-and-out-quickly reconstruction that is not particularly concerned about achieving cosmesis.”
Option No. 3 was the radial forearm free flap. “This would provide a means to line the entire involved area with a thin, fasciocutaneous vascularized tissue capable of facilitating good cosmesis and being less vulnerable than a rectus free flap to complications,” Azizzadeh explains.
Performing the Anastomosis
Azizzadeh harvested the free flap by taking skin, soft tissue, and underlying artery and veins from the radial aspect of the forearm. The placement process began with one of the surgeons—ear, nose, and throat cancer specialist Babak Larian, MD—exposing the patient’s neck artery and vein to create corresponding receptors for Azizzadeh’s microvascular anastomosis of the free flap’s arterial and venous tissues.
“Key to the success of anastomosis— as with any head and neck free flap reconstruction—was proper positioning of the artery and vein so that they would each lay in as normal and natural a manner as possible, without kinking onto the neck vessels,” Azizzadeh explains. “Kinking is the most frequent cause of free-flap failure.
“To make sure I was able to lay the artery and vein smoothly, I started the attachment of the distal aspect of the free flap onto the most distal portion of the reconstruction. Afterward, when I was ready to lay the free flap over the area of coverage, I worked from the superior aspect of the orbit, then curved down to the deep part of the orbit into the sinus area, moved along to the infratemporal fossa and finally out onto the skin.” (See Figure 4, page 68.)
Soon after Azizzadeh had finished his work, a temporary dental prosthesis was placed so that the patient would be able to drink, eat, and speak in the days following surgery.
The in-hospital recovery period was surprisingly brief, considering the extensiveness of the operation. “The patient was discharged directly to his home 5 days postoperatively,” Azizzadeh says.
The team then waited approximately 8 weeks after surgery before placing the midface prosthesis. “We wanted to be sure that we had given the patient enough time to heal before putting it in,” Azizzadeh says, mentioning their concern with regard to healing in light of the patient’s prior involvement with radiation therapy.
 |
| See also “Resurfacing the Face” by Rich Smith in the January 2006 issue of PSP. |
Azizzadeh has seen the patient a few times in the months since surgery. “I like to follow a patient who has undergone this type of procedure for at least 1 year because the flaps sometimes contract and then that requires a minor touch-up procedure,” he says. “I don’t anticipate that happening to this patient, given how well he has been doing so far. In fact, the cosmetic result turned out really, really great—even better than I thought it would.”
Richardson concurs. “The defect looks quite good,” he says. “Also, and most importantly, there has been no sign of cancer recurrence. The patient is very satisfied with his quality of life, and he is doing very well.”
Rich Smith is a contributing writer for Plastic Surgery Products. For additional information, please contact [email protected].
The Surgical Lineup
 |
| Babak Azizzadeh, MD, (left) and Madison F. Richardson, MD |
The operation on the 62-year-old maxillary sinus cancer patient at Cedars-Sinai Medical Center, Los Angeles, was performed by a team of specialists that consisted of an anesthesiologist, head and neck surgeons, a neurosurgeon, a prosthodontist, and plastic reconstructive surgeons. Here are the key members of that team.
- Madison F. Richardson, MD. Board certified in otolaryngology–head-and-neck surgery, Richardson was the lead surgeon on this case. His Beverly Hills practice is one of the largest and most distinguished in the city, and he was named an America’s Top Doctor by the Center for the Study of Services. Richardson began his career at Walter Reed Army Medical Center, where he later rose to the post of chief of the head and neck surgery department, as well as the director of the head and neck surgical training program. Since entering private practice, Richardson has held surgery department faculty positions in the schools of medicine at the University of California, Los Angeles and the University of Southern California.
- Babak Azizzadeh, MD. Azizzadeh is a facial plastic surgeon exclusively devoted to aesthetic and reconstructive surgery of the face, eyes, and nose. He was asked onto Richardson’s team because he and the ear, nose, and throat physician have enjoyed a history of working together on such cases and have developed an enviably cohesive relationship. Azizzadeh is director of the Center for Facial and Nasal Plastic Surgery in Los Angeles. He earned his MD degree from the UCLA School of Medicine, completed a 6-year residency program in head and neck surgery–facial plastic surgery at UCLA Medical Center, and capped his training with a subspecialized fellowship in advanced facial plastic and reconstructive surgery at Harvard Medical School. Today, he is an assistant clinical professor at UCLA and an author of numerous frontier-expanding articles in peer-reviewed journals.
- Kami Parsa, MD. As an oculoplastic surgeon, Parsa was invited onto Richardson’s team to assist with the orbital reconstruction part of the case in which the removed eyeball was returned to its socket and made to look normal again. Parsa belongs to the same private practice as Azizzadeh—Beverly Hills-based Alpha Surgical Group. Parsa is a member of the American Academy of Ophthalmology and is an assistant clinical professor at USC School of Medicine.
- Babak Larian, MD. Another member of Alpha Surgical Group, Larian is the associate director of the Head and Neck Cancer Center at Cedars-Sinai and director of the medical center’s head and neck tumor board. He is an assistant clinical professor of surgery at UCLA’s David Geffen School of Medicine.
Earlier, the patient underwent radiation therapy administered by David Rickles, MD, from the Beverly Hills Radiation Oncology Group. Eli Gabayan, MD, is a medical oncologist (and member of the Cedars Sinai tumor board) who supplemented the radiation therapy with chemotherapy.
—RS




