|
November 2006 ushered in a new age in breast augmentation in the United States, following approval by the FDA for specific types of silicone gel-filled breast prosthetic devices.
For nearly 15 years prior to this, cosmetic breast surgeons were restricted to the use of saline-filled implants for basic augmentations or for women who did not fit into the reconstructive category required by an adjunct study.
The choices afforded to women 30 years prior to the 1992 moratorium on silicone gel breast implants were immediately limited.
Currently, women over 22 years of age have reacquired the freedom of choice between silicone gel-filled or saline-filled breast implants.
Choice creates dilemmas for the patient and definitely for the surgeon, who must try to make sense of the many past studies that outline the differences between saline-filled and silicone gel-filled breast implants.
When reviewing literature, one must also keep in mind that results, even those from as recently as 5 years ago, may not accurately represent the current state of the technology—the latest implant products are not comparable to older designs.1,2
Today’s breast implants come in multiple styles (shapes, texturing, etc) that undoubtedly skew the results.
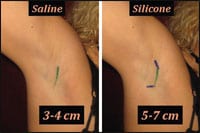 |
| Figure 1. The incision for saline implants is shorter, as depicted by the green line in this patient’s right axilla. Blue extensions of the incision line demonstrate the longer incision length required for placement of gel-filled implants. A posterior curvature of this incision helps prevent the incision from tearing during gel implant placement. |
In addition, some studies are potentially unreliable and prejudicial, as a result of competition between two major implant companies with considerable financial interest in getting their products to market as soon as possible.
The Inamed (now Allergan) and Mentor breast implant divisions had to go through a very long and arduous process that hopefully resulted in superior products that are very safe to use.3-6,7
Giving adequate informed consent to the breast augmentation patient was much easier prior to the FDA’s approval of silicone gel implants—the FDA’s new, complex options and recommendations require the physician to spend more time with patients, explaining risks, benefits, and options for breast augmentation.
For example, in addition to the 22-year-old age threshold mentioned earlier, you must conduct an initial 3-year MRI follow-up study of the patient’s breast, followed by additional breast MRIs every 2 years.
The surgeon must explain in clear terms the other many advantages and disadvantages of each implant type, along with general surgical risks.
UNDERSTANDING THE DIFFERENCES
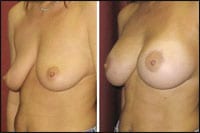 |
| Figure 2. Before and 4 months after placement of silicone gel-filled implants in a subglandular plane. The 34-year-old patient had near grade 2 ptosis preop and preferred no major lift, a “natural look and feel,” and minimal downtime. Therefore, a gel-filled prosthesis offered a good alternative to a vertical mastopexy or submuscular saline implants. |
Leakage can occur in either saline-filled or silicone gel-filled implants. Final studies on rates have yet to be determined, but leakage appears to be higher in textured implants when compared with smooth.
Historically, leakage has been higher in silicone gel implants compared with saline.8-13 Leakage of a saline implant can easily be determined clinically, whereas leakage from a gel-filled implant may be difficult to determine—even an MRI study may not definitively demonstrate a rupture.
In addition, FDA recommendations for silicone gel-filled implants must be discussed with the patients, as well as the fact that, typically, the entire procedure is not covered by most insurance companies.
Capsular contracture can occur with either type of implant but has historically been much higher with gel-filled prosthesis compared with saline-filled implants. The recently approved gel-filled implants appear to have a lower capsular-contracture rate than previous generations of silicone gel-filled implants.
However, the new gel implants need more study before we know the long-term rates of capsular contracture.
Rates of capsular contracture vary by study and implant type, but average in the range of 1% to 2% per year, progressively, and are likely well over 10% after 10 years.14-18
The two foremost benefits of silicone gel-filled implants over saline are a “more natural feel” and “decreased rippling.” In addition, these benefits may be more dramatic in patients with thinner breast tissues, or when using larger implants.
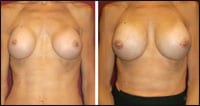 |
| Figure 3. Before and 6 months after replacement of small saline implants with wider silicone gel-filled implants. The 48-year-old thin patient was unhappy with implants that were too narrow and did not give her the “feel, size, or cleavage” that she desired. Her case is classic and problematic because of the extremely thin and adherent tissue over her sternum that will allow rippling with wider implants of any type, but usually less so when using smooth gel-filled devices. |
Rippling is less common in silicone gel implants but may still occur in the current gel-filled implants approved by the FDA. Overall, the satisfaction rate for both types of implants is routinely more than 90%.
Patients who choose silicone gel-filled implants must understand that the “cohesive gel” implants approved by the FDA can still leak even though they are less “watery” than older gel implants. “Gummy bear” (form stable)-style implants are not yet approved by the FDA and, therefore, are not readily available.
Gel-filled implants cost approximately twice as much as saline. Therefore, the final surgical cost to the patient will be higher. If revisions or replacements are eventually needed, the patient will have to spend more money in addition to any costs involved in complying with FDA follow-up recommendations.
Slightly smaller incisions are required for saline-filled implants compared with gel-filled implants.
No implant is meant to last a lifetime. The vast majority of patients will need additional surgery related to their implants sometime later in life—approximately 20% will see that happen within 10 years of the original surgery.
No one knows how well the best-quality implants will hold up after 10, 20, or even 30 years. Studies are ongoing to determine the life cycle of the latest implant types, and previous studies likely do not accurately reflect that life cycle.8-13
INCISIONS AND SURGICAL TECHNIQUE
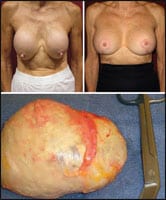 |
| Figure 4. Before and 6 months after removal and replacement of 30-year-old silicone gel-filled implants, due to complaints of hardness and distortion in appearance. The 73-year-old thin patient underwent simultaneous complete capsulectomies and placement of new gel-filled implants in a submuscular plane along with vertical mastopexies. One of the implants shown was removed with the capsule fully intact, demonstrating the distortion created from severe capsular contracture and associated calcification. |
There are four major choices with regard to incision placement for standard breast augmentation: Periareolar, Inframammary, Transumbilical, and Transaxillary.
All four major incisions can be used to place saline-filled implants above or below muscle. A fifth incision choice is based on existing scars or even an abdominoplasty incision performed simultaneously—this choice can be used for insertion of saline or silicone gel implants.
The companies that produce implants recommend against the use of the Transumbilical approach for placing silicone gel-filled implants, as it may disrupt the integrity of the implant shell.
Regardless of the incision choice, the overall length of the incision depends on tissue quality and whether or not a saline- or gel-filled implant is being used.
Incisions for saline implants can be much shorter (3 cm to 4 cm in length) than the incisions normally used for placement of silicone gel implants (which are usually 5 cm to 7 cm in length) (Figure 1).
Proper pocket development and adequate tunnel size toward the pocket are more important than the incision length, in order to avoid trauma to the gel implant upon insertion.
Sensible yet firm retraction with ample tissue elevation of tissue deep within the pocket is required for final bimanual silicone gel implant placement.
Saline implants require the same meticulous pocket development, but not nearly the level of retraction of the tunnel for implant placement.
The Transaxillary technique for placement of gel-filled implants is even more technique-sensitive to avoid unnecessary trauma to adjacent issues, compared with the placement of a rolled-up, empty saline implant.
When Silicone Gel-filled Implants Can Be Very Beneficial
- Patients who do not desire mastopexy but have moderate ptosis that may risk a double bubble deformity if a submuscular approach is taken. These patients could benefit from a subglandular placement of an implant. Moreover, silicone gel implants often feel more natural and ripple less than the saline-based variety, particularly when placed in this subglandular tissue plane (Figure 2).
- Patients who have previous saline implants with rippling; in particular, thin patients who have a wide cleavage space and desire larger implants and more cleavage (Figure 3).
- Patients who have lived with older silicone gel implants for many years and desire a replacement (Figure 4).
- Complicated or revision cases in which the amount of residual muscle is unknown. Often, after scar tissue is excised, a less than desirable amount of tissue remains that could compromise the result. A saline-filled implant would be more likely to show rippling in this situation. The use of a gel-filled implant in this situation may improve the aesthetic outcome and the way the breast feels to the patient.
Silicone “Backlash”
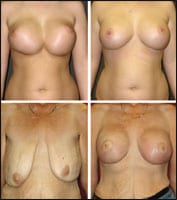 |
| Figure 5. Top: Before and 6 months after correction of synmastia by pocket reconstruction and replacement of saline implants with silicone gel-filled implants. Bottom: Before and 6 months after a simultaneous mastopexy and silicone gel implant augmentation of this 63-year-old who lost more than 130 pounds after gastric bypass surgery. Both patients benefited from gel-filled implants because of compromised tissue quality. |
The use of silicone gel-filled implants can be advantageous over the use of saline, yet one could argue that they are not worth the risks and that saline implants would do “just fine.”
In addition, physicians who had performed silicone gel augmentations for many years may be somewhat reluctant to jump back on the silicone bandwagon—and for good reason, particularly as saline implants have proven to be very safe, with satisfaction rates above 93% in most studies.11,13
Nevertheless, the trend among physicians is the increased use of gel-filled implants.
Based on conversations with top representatives of both Allergan and Mentor, the sale in pure numbers of saline-filled versus gel-filled implants for each company is now near 50% across the United States.
Two years ago, before the FDA allowed widespread usage of gel implants, the percentage of gel implants being used in my practice was just over 12%. Prior to 2005, the percentage of gel-filled implants was less than 5%.
Just over a year later, this same practice completed breast implant surgery in more than 2,600 patients—most of which were saline-filled products—and is presently experiencing a 71% rate for gel-filled implants.
Admittedly, this group of patients has a mean age of 38 for breast augmentation, and a very high percentage of our patients are either revision-type cases or women with significant ptosis requiring mastopexy.
An ever-growing number of women who have experienced massive weight loss following bypass surgery are overwhelmingly choosing silicone gel breast implants in our practice (Figure 5).
Regardless of practice location or practice type, the use of silicone gel-filled implants is growing whether we like it or not.
History will shows what best restores or augments breast volume with the highest level of satisfaction and lowest rates of complications. Society will also play a role in what the next generation of breast-implant patients choose to keep inside their bodies for aesthetic purposes.
As surgeons, we have to continue to adapt and strive to provide our patients with the information they need in order to make informed and reasonable decisions.
Angelo Cuzalina, MD, is in private practice in Tulsa, Okla, and is the director of a Cosmetic Surgery Fellowship program accredited by the American Academy of Cosmetic Surgery. He was elected to the Board of Trustees for the AACS in 2005 and currently serves as treasurer. Cuzalina is board certified by the American Board of Cosmetic Surgery, has authored numerous book chapters and articles, and taught numerous ACCME-approved live surgery workshops in aesthetic surgery. He can be reached at [email protected].
PHOTOS COURTESY OF ANGELO CUZALINA, MD.
References
- Marotta JS, Goldberg EP, Habal MB, et al. Silicone gel breast implant failure: Evaluation of properties of shells and gels for explanted prostheses and meta-analysis of literature rupture data. Ann Plast Surg. 2002;49:227-242;discussion 242-7.
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: A critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96:1521.
- Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117:757;discussion 768.
- Mentor Corp. Product insert: Saline-filled breast implant surgery; making an informal decision. Santa Barbara, Calif: Mentor Corp; 2004.
- Allergan Corp. Product insert: Allergan core study. Irvine, Calif: Allergan Corp; 2006.
- Mentor Corp. Product insert. Mentor core study results. Santa Barbara, Calif: Mentor Corp; 2006.
- Rohrich RJ. The FDA approves saline-filled breast implants: What does this mean for our patients? Plast Reconstr Surg. 2000;106:903.
- De Camara DL, Sheridan JM, Kammer BA. Rupture and aging of silicone gel breast implants. Plast Reconstr Surg. 1993;91:828;discussion 835.
- Cairns TS, de Villiers W. Capsular contracture after breast augmentation: A comparison between gel- and saline-filled prostheses. S Afr Med J. 1980;57:951.
- Rohrich RJ, Adams W Jr, Beran SJ, et al. An analysis of silicone gel-filled breast implants; Diagnosis and failure rates. Plast Reconstr Surg. 1998;102:2304;discussion 2309.
- Gutowski KA, Mesna GT, Cunningham BL. Saline-filled breast implants: A Plastic Surgery Educational Foundation multicenter outcomes study. Plast Reconstr Surg. 1997;100:1019.
- Holmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003;138;801.
- Cunningham BL, Lokeh A, Gutowski KA. Saline-filled breast implant safety and efficacy: A multicenter retrospective review. Plast Reconstr Surg. 2000;105:2143;discussion 2150.
- Heden P, Bone B, Murph DK, Slicton A, Walker PS. Style 410 cohesive silicone breast implants: safety and effectiveness at 5 to 9 years after implantation. Plast Reconstr Surg. 2006;118:1281.
- Heden P, Nava M, van Tetering JPB, et al. Prevalence of rupture in Inamed silicone breast implants. Plast Reconstr Surg. 2006;118:303;discussion 309.
- McLaughlin JK, Lipworth L, Murphy DK, Walker PS. The safety of silicone gel-filled implants: a review of the epidemiologic evidence. Ann Plast Surg. 2007;59:569.
- Cunningham B. The Mentor study on contour profile gel silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120:33S-39S.
- Spear SL, Murphy DK, Slicton A, Walker PS. Inamed silicone breast implant US study group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120:8S-16S;discussion 17S-18S.