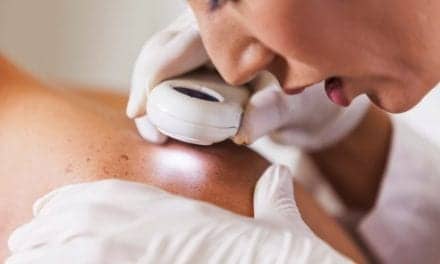
Clinical trial results show improved diagnostic accuracy with a handheld, noninvasive molecular imaging system used to assess difficult-to-characterize skin lesions.
In a clinical trial, researchers at Orlucent Inc reported positive results related to the accuracy of the company’s Skin Fluorescent Imaging (SFI) System, a noninvasive, handheld molecular imaging device designed for point-of-care assessment of suspicious skin lesions and early melanoma-related activity.
The results of the study conducted by Orlucent, a biotechnology company developing solutions for the non-invasive detection of melanoma activity, were published in the Journal of the American Academy of Dermatology International.
The study found that the use of Orlucent’s SFI noninvasive, point-of-care molecular imaging method was associated with improved diagnostic accuracy in classifying difficult-to-characterize moles, with findings suggesting potential to reduce unnecessary biopsies and support earlier identification of aggressive cancers.
“This is a major step forward for non-invasive skin cancer diagnostics,” says Douglas Grossman, MD, of Utah’s Huntsman Cancer Institute and the study’s lead author, in a release. “Skin Fluorescent Imaging lets doctors see melanoma molecular activity in the skin, which may help reduce unnecessary biopsies and improve early detection.”
Melanoma is the most lethal form of skin cancer, often caused by ultraviolet exposure from sunlight or indoor tanning and can progress to metastatic disease if not detected early. Today, clinical evaluation relies largely on subjective visual assessments of suspicious moles using the ABCDE criteria (asymmetry, border, color, diameter, and evolution).
However, many suspicious or “atypical” moles are not melanoma, and conversely, some melanomas lack the classic criteria, making these moles difficult to classify. To avoid missing an early melanoma, dermatologists follow a “when in doubt, cut it out” approach, performing an estimated 5 to 10 million biopsies annually that lead to the identification of roughly 100,000 melanomas, “resulting in substantial overtreatment, scarring, and unnecessary patient anxiety,” according to a release from the company.
SFI aims to deliver an objective measure of cellular activity for a mole to reduce reliance on subjective visual assessment by healthcare professionals.
“This technology works like a molecular map for moles, revealing changes before they are visible to the eye and giving doctors more confidence about whether to biopsy a mole or monitor it,” says Oregon Health & Science University’s Sancy Leachman, MD, senior author and study investigator, in a release.
Study Results
The clinical trial examined the use of SFI in 240 patients with difficult-to-diagnose moles (not obviously melanoma or benign), across six academic and community dermatology clinics in California, Utah, and Oregon. Among the key findings:
- SFI detected oncogenic activity in 100% of melanoma cases.
- SFI cutoff scores of 5 (where 5-10 is considered positive) or 7 (where 7-10 is considered positive) demonstrated sensitivities (true positive detection rate for melanoma, melanoma in situ and high-grade dysplasia) of 93% (5 cutoff) and 87% (7 cutoff) and specificities (true negative rate for no or low-grade dysplasia) of 77% (5 cutoff) and 91% (7 cutoff), respectively.
- SFI exhibited excellent overall diagnostic accuracy or Area Under the Curve (where 1 is perfect) at 0.907 (95% CI 0.864-0.951, P <.0001).
- When compared to the use of dermoscopy (a polarized magnifying glass), SFI performance showed sensitivity of 89% vs 53 %, and specificity of 94% vs 61%.
SFI’s technology works by identifying the presence of αvβ3 integrin, which is expressed in moles undergoing oncogenic tissue remodeling with the potential for metastatic disease at the earliest stage. The SFI test procedure includes application of a fluorescent peptide dye that binds to this αvβ3 integrin. A handheld imaging device captures the signal created, and Orlucent Mole Analytics Software, powered by artificial intelligence and machine learning, analyzes it in real time.
The SFI System has received Breakthrough Device Designation by the US Food and Drug Administration and is currently completing additional trials to support regulatory approval.
“Our goal is to make advanced molecular insight simple and accessible,” says Catherine Shachaf, PhD, Orlucent’s co-founder and chief scientific officer, in a release. “SFI helps dermatologists move beyond surface features and understand what’s happening inside a mole, making the invisible visible and potentially catching aggressive changes earlier than ever.”
ID 176159245 | Dermatologist Mole © Aleksandr Malikov | Dreamstime.com