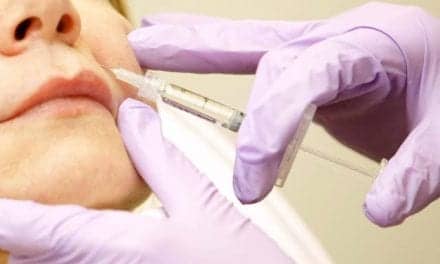
The FDA has approved a clinical trial of Botox as a treatment for vaginal spasms that can block sexual intercourse and gynecological examinations.
The condition, known as vaginismus, affects 1% to 7% of women worldwide — “About the same percentage as men with erectile dysfunction,” according to Peter T. Pacik, MD, FACS, of Manchester, NH, who will lead the study. It is “the most common cause of unconsummated marriages,” according to his press release announcing the FDA action.
Pacik’s account of the prevalence of vaginismus isn’t shared by everyone. A medical information Web site of the National Institutes of Health calls it “an uncommon condition” and adds, “The exact number of women who have this problem is unknown.”
The makers of Botox, Irvine, Calif-based Allergan, are “aware but have not provided monetary or financial funding for the study, which is not initiated by Allergan,” says company spokeswoman Caroline Van Hove.
In the past five years, before getting the official FDA approval, Pacik said he has treated more than 70 vaginismus sufferers with a combination of Botox injections, local anesthesia, and a vaginal dilator, applied gradually.
More.
[Source: The Orange County Register]