|
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), are potential complications of plastic surgery procedures. Often presenting with subtle signs and symptoms, the recognition and diagnosis of DVT and PE may be delayed, thereby leading to significant morbidity and mortality.
It is estimated that PE accounts for 100,000 to 200,000 deaths annually in the United States.1 Approximately 5% of all perioperative deaths are attributable to PE.2 In plastic surgery procedures, PE is the leading cause of death following liposuction,3 and it is estimated that the incidence of DVT following abdominoplasty is as high as 1.2%.4 Interestingly, rhytidectomy is also associated with a 0.35% incidence of DVT and a 0.14% incidence of PE.5
It is important for the surgeon to realize that DVT is commonly asymptomatic,6 and that the majority of fatal PEs are not suspected prior to death.7 In addition, the rate of PE in patients with untreated proximal (above-knee) DVT approaches 50%. Fortunately, this rate may be reduced to less than 5% with current treatment algorithms.4
With general anesthesia and increased surgical times becoming more commonplace, it is imperative that plastic surgeons recognize the risk factors for VTE. In addition, surgeons must also understand the prevention strategies aimed at reducing VTE formation, the early signs and symptoms of VTE, and the evaluation of patients suspected of having VTE.
Finally, a knowledge of treatment options is critical in determining the balance between managing the risks of anticoagulation and postoperative bleeding. This article is intended to address these issues to help reduce the incidence of this potentially devastating complication.
Clinical Risk Factors
Because VTE is common, especially in hospitalized patients, the surgeon must recognize and elicit a patient’s risk factors preoperatively. Interestingly, in a multicenter study, the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) found that 60% to 70% of autopsied hospitalized patients had one or more pulmonary emboli present.8
Awareness as to the frequency of VTE, and the possibility of preexisting VTE, is a first step in reducing complication rates. The next step—recognizing known risk factors—is essential for designing prophylactic strategies.
Known risk factors include:
- age over 40 years,
- obesity,
- previous history of VTE,
- cancer,
- prolonged bed rest (more than 5 days),
- major surgery,
- congestive heart failure,
- varicose veins,
- fracture (hip or leg),
- estrogen treatment,
- stroke,
- multiple trauma,
- childbirth, and
- myocardial infarction.
Other conditions are also associated with an increased risk of VTE (see “Risk Factors for DVT and PE”). The plastic surgeon should inquire as to these issues during the preoperative consultation.
In addition to identifying risk factors, stratification of these factors may be performed based upon their relative risk (see “Strong, Moderate, and Weak Risk Factors for VTE”).
Clinical Risk Assessment
In an effort to better quantify risk, Davison et al proposed an assessment model involving a four-step scoring system.10 These steps entail tallying a list of both “exposing” risk factors and “predisposing” risk factors with the intention of determining an ultimate risk assignment. Risk assignment is then characterized as low risk, moderate risk, high risk, and highest risk. Recommendations for prophylaxis are based upon a patient’s designated risk stratification.
When clinically appropriate, all patients are instructed to ambulate three times daily in the postoperative period. While this may be sufficient for low-risk patients, moderate- and high-risk patients are also instructed to wear mechanical compression devices. For patients deemed highest risk, mechanical compression devices—in addition to pharmacologic prophylaxis—may be indicated.
It is recommended that the risk of VTE be discussed preoperatively with patients considered high-risk based upon either historical risk factors or for patients undergoing procedures known to carry a high risk, such as belt lipectomy. In addition, for those patients considered high- or highest-risk, thought should be given to performing these procedures in an acute care setting.
Preventive Measures
First and foremost, the plastic surgeon should inquire preoperatively as to the individual patient’s risk factors for VTE. Following this, a strategy for prophylaxis should be designed (Figure 1).
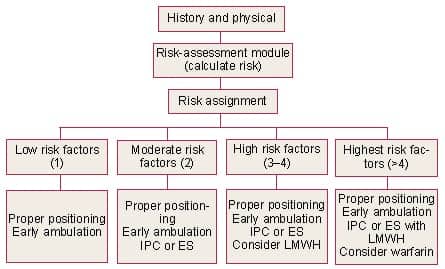 |
| Figure 1. Algorithm for VTE prevention in plastic surgery patients. IPC, intermittent pneumatic compression stockings; ES, elastic compression stockings; LMWH, low molecular weight heparin.10 |
Fortunately, prophylaxis—either mechanical or pharmacological—is effective in reducing the risk of VTE in surgical patients. These risk-reduction maneuvers may begin prior to the patient entering the operating room, and include graded elastic compression or pneumatic compression. In addition, once in the operating room, positioning on the operating table may be helpful in promoting venous flow through the popliteal veins. A pillow placed under the knees, with the knees flexed at 5°, will assist in this endeavor.10
Early ambulation in the postoperative period should also be considered. For patients in the low-risk group, positioning and ambulation may be sufficient.10
For patients in the moderate- and high-risk categories, pneumatic compression is recommended. Placement of these devices should occur prior to induction of general anesthesia. Compression devices are postulated to work by an increase in fibrinolytic activity as well as increasing venous return.11 Interestingly, compression devices may be placed on the upper extremity if the legs are prepped into the sterile field.
Finally, for those patients in the highest-risk group, consideration should be given to the addition of pharmacologic prophylaxis. This includes low-dose unfractionated heparin or low molecular weight heparin (LMWH). There may be certain advantages to the use of LMWH over unfractionated heparin—the option of once-daily dosing and a lower incidence of heparin-induced thrombocytopenia. One disadvantage is the increased cost of LMWH. Obviously, when anticoagulants are used, the risk of bleeding must be weighed against the risk of VTE.
Assessment
Unfortunately, the diagnosis of VTE is not necessarily straightforward. Symptoms may be similar to other conditions, such as myocardial infarction, pneumonia, or congestive heart failure, and there may be no symptoms, as mentioned above.
Although clots may resolve spontaneously, thrombosis must be treated. Roughly one third of untreated below-knee DVTs will extend above the knee within 1 week, and the rate of PE in patients with untreated proximal DVT is almost 50%. Besides that, approximately 10% of patients with an acute PE die within 30 to 120 minutes of embolization.4
The signs and symptoms of DVT include leg pain, swelling, tenderness, warmth, erythema, a positive Homan’s sign, and palpable thrombosed veins in the legs. The symptoms of PE may be nonspecific and may be acute or develop over days (see “Signs and Symptoms of DVT and PE”, below).
These symptoms must be differentiated from other potential cardiopulmonary sources. Additional signs of acute PE include tachypnea, tachycardia, crackles, diaphoresis, and fever. Once again, these signs have multiple potential etiologies in the postsurgical patient.
Alternative diagnoses such as pain, dehydration, infection, and so forth are often considered. However, when there is high suspicion for PE, it should be investigated. Neither DVT nor PE can be diagnosed clinically without an imaging study. It is estimated that clinicians accurately diagnose PE only 30% of the time when based on clinical criteria alone.12
Diagnosis of DVT
D-dimer is a derivative that arises from the breakdown of cross-linked fibrin and may be helpful in the evaluation of DVT. Although laboratory tests cannot definitively confirm DVT or PE, a negative D-dimer in the setting of a low clinical suspicion helps to exclude the diagnosis. Conversely, although a positive D-dimer suggests that VTE may be present, other potential etiologies exist, such as infection, cancer, and trauma. Therefore, when the D-dimer is positive, additional testing is warranted.
Arterial blood gas may be normal in the presence of PE and is of little value in the diagnosis of PE. It is, however, helpful in determining the need for inspired oxygen. Other tests, such as electrocardiography and chest radiographs, may also be useful adjuncts in the evaluation process.
Once again, PE cannot be diagnosed on the basis of these tests alone. However, these tests may be helpful in assessing for alternative diagnoses such as myocardial infarction, pericarditis, or pneumonia.
Imaging Studies
Ultrasound is the most commonly performed imaging study. It is readily available, noninvasive, and accurate. Ultrasound is considered to be greater than 90% sensitive in the presence of symptomatic DVT. However, in the absence of symptoms, the sensitivity and specificity of ultrasound decreases. Ultrasound may therefore be considered more of a screening exam for DVT.
When considering the diagnosis of PE, additional examinations are required. Pulmonary angiography is considered the gold standard in the diagnosis of acute PE; however, it is invasive. Noninvasive modalities include ventilation–perfusion (V/Q) scan and spiral computed tomography (CT).
V/Q scan had been the mainstay of imaging, but its utility is greatest at the extremes of ratings. In other words, high-probability scans in conjunction with high levels of clinical suspicion may accurately predict PE 87% of the time.
Similarly, a normal study in association with a low level of suspicion is associated with PE less than 5% of the time.4 Intermediate readings require additional imaging modalities, such as pulmonary angiography.
An additional noninvasive imaging study is the spiral CT. However, a normal spiral CT is associated with a lower sensitivity than a normal V/Q scan. The sensitivity for acute PE ranges from 53% to as high as 100% with spiral CT.12
Treatment
In stable patients, unfractionated heparin or LMWH can be used for the short-term treatment of VTE. Because the full therapeutic effect of vitamin K antagonists such as warfarin requires 5 to 7 days, concurrent administration of unfractionated heparin or LMWH is recommended until the international normalized ratio is stable and greater than 2.0.13
LMWH may have several advantages over unfractionated heparin. They include lower rates of heparin-induced thrombocytopenia, the ability to administer LMWH subcutaneously, and eliminating the need for monitoring activated partial thromboplastin time. In addition, LMWH has been shown to be at least as effective and safe as intravenous unfractionated heparin as an initial treatment for patients with VTE.13
Because LMWH can be administered subcutaneously and routine laboratory monitoring is not required, there is the added advantage of home treatment in patients with DVT. Whereas outpatient treatment of DVT is more common, it is limited to those patients least likely to suffer an adverse event.
The decision for outpatient treatment is individualized to the patient. Patients not considered eligible for outpatient treatment include those who have had two or more previous episodes of DVT or PE, or VTE within the preceding 2 years. Also ineligible are those with:
- current active bleeding;
- active peptic ulcer disease;
- familial bleeding disorder;
- concurrent symptomatic PE or suspected PE;
- previous treatment with heparin for more than 24 hours;
- likelihood of noncompliance;
- life expectancy of less than 6 months;
- overt post-thrombotic syndrome;
- inability to use LMWH because of a coexisting condition such as stroke;
- age less than 18 years;
- pregnancy; or
- known deficiency of antithrombin III, protein C, or protein S.
Appropriate consultation with a medical specialist, such as an hematologist, should be considered as well.
Long-Term Treatment
Studies have shown that cessation of treatment prior to 3 months of therapy results in an increased risk of extension of the thrombosis or recurrent VTE.14 The American College of Chest Physicians recommends long-term treatment with warfarin for 3 months or longer. Warfarin is the most commonly used anticoagulant and reduces the rate of recurrent VTE by 90% during treatment.
The decision as to duration of therapy must consider the patient’s bleeding risk in addition to known risk factors. If a patient’s VTE is associated with known, transient risk factors (such as surgery or trauma), 3 months of treatment after the risk factor is no longer present may be sufficient. Patients with permanent risk factors, such as protein C deficiency, may require treatment indefinitely. For patients with idiopathic VTE, guidelines recommend 6 to 12 months of treatment.
VTE remains a possible complication of surgery, and should be discussed preoperatively with patients who are considered to be at increased risk. Besides that, the plastic surgeon needs to be aware of not only risk factors for VTE but also prevention strategies.
Whereas prevention remains the goal, awareness of the often subtle signs and symptoms of VTE is also critical. If the diagnosis of VTE is suspected, appropriate imaging studies should be performed. Finally, in the event of an affirmative diagnosis, appropriate treatment must be initiated in a timely fashion. As plastic surgeons, our goal is to offer our patients the highest-quality care in the safest manner.
Loren S. Schechter, MD, FACS, is in private practice in Morton Grove, Ill, and serves as division director of plastic surgery at Lutheran General Hospital in Park Ridge, St Francis Hospital in Evanston, and Rush North Shore Medical Center in Skokie. He is certified by the American Board of Plastic Surgery and teaches at the University of Chicago Pritzker School of Medicine. He can be reached at (847) 967-5122 or .
References
- Dalen JE. Pulmonary embolism: What have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest. 2002;122:1440–1456.
- Anderson FA, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates for deep vein thrombosis and pulmonary embolism. The Worcester DVT study. Arch Intern Med. 1991;151: 933–938.
- De Jong RH, Grazer FM. Perioperative management of cosmetic liposuction. Plast Reconstr Surg. 2001;107:1039–1044.
- Most D, Kozlow J, Heller J, Shermak M. Thromboembolism in plastic surgery. Plast Reconstr Surg. 2005;115:20e–30e.
- Reinisch JF, Bresnick SD, Walker JWT, Rosso RF. Deep venous thrombosis and pulmonary embolus after facelift: A study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107:1570–1575.
- Tapson VF. The diagnosis of acute venous thromboembolism. Dis Mon. 2005;182: 569–574.
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108: 978–981.
- The PIOPED Investigators. Value of ventilation/perfusion scan in acute pulmonary embolism: Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753–2759.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I-9–I-16.
- Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114:43e–51e.
- Comerota AJ, Chouhan V, Harada RN, et al. The fibrinolytic effects of intermittent sequential calf compression: Mechanisms of enhanced fibrinolyis. Ann Surg. 1997;226: 306–313.
- Tapson VF. Identifying patients with deep vein thrombosis or pulmonary embolism: Current standards and future directions. In: Tapson VF, ed. Treatment of Venous Thromboembolism (monograph). Elsevier Press, May 2006:2–10. Available at: www.thrombosisclinic. com/11/39/5128/5155/270103_web_5-24_final_revise1.pdf. Accessed May 14, 2007.
- Goldhaber SZ. Outpatient treatment of deep vein thrombosis or pulmonary embolism: Feasible and safe, but who qualifies? In: Tapson VF, ed. Treatment of Venous Thromboembolism (monograph). Elsevier Press, May 2006:11–20. Available at: [removed]www.thrombosisclinic.com/11/39/5128/5155/270103_web_5-24_final_revise1.pdf[/removed]. Accessed May 14, 2007.
- Menajovsky LB. Long-term treatment of venous thromboembolism: Management principles. In: Tapson VF, ed. Treatment of Venous Thromboembolism (monograph). Elsevier Press, May 2006:39–48. Available at: [removed]www.thrombosisclinic.com/11/39/5128/5155/270103_web_5-24_final_revise1.pdf[/removed]. Accessed May 14, 2007.