|
With the emerging importance of body image, changes in societal expectations, and the increasing acceptance of aesthetic surgery in the United States, there has been a steady increase in the popularity of breast-augmentation surgery.
However, American women’s health options and choices regarding aesthetic (and to a large extent, reconstructive) breast surgery have been restricted for more than 15 years. The 1992 FDA “ban” on silicone gel breast implants was viewed askance by women in the rest of the world who have selected silicone over saline by a greater than nine-to-one ratio during the same period.
Saline implants—with their less natural feel and shape than silicone—essentially have relegated breast augmentation to a volume-enhancement operation. These devices enlarge breasts, but they offer little option for the aesthetic considerations of form and proportion—which truly define female beauty. Thus, the FDA’s 2006 reapproval of silicone gel breast implants was the dawn of an important new day for American women. It gave them better options to enhance their form, proportion, and femininity.
Silicone breast implants that were first developed in the 1960s had “teardrop” shapes. They had a thick shell filled with a thick gel, and they were backed by Dacron patches to “keep them in place.”
These first-generation devices gave way in the 1970s to round, thin-shelled devices filled with a thin liquid silicone gel. These second-generation devices were developed to feel soft, but did not hold up over time, and their high failure percentage caused most of the problems women experienced regarding leakage and breakage. These devices were one of the main causes of the ultimate FDA “ban.”
Confirmation of Safety
It has taken many years to prove the safety of silicone gel, and silicone breast implants are now the single most studied medical device in the history of medicine. Beginning in the mid 1980s, “good manufacturing practices” (GMPs) were applied to breast-implant production, including standardization of materials (stronger shells and improved gels), in-line testing, and other strict safety protocols. These third-generation devices were better and stronger than their predecessors.
The silicone gel breast implants recently approved by the FDA are fourth-generation implants, different from those removed from the market in 1992. They are safer (as confirmed by compelling data generated by multiple significant scientific studies) and stronger (a result of more standardized manufacturing processes), and they have been quickly accepted by American women.
 |
| Figure 1. A form-stable highly cohesive silicone gel implant (left) maintains its shape within the body, whereas a non–form-stable implant collapses to some degree following implantation. |
The cohesive silicone gel in these implants is similar to a thick hair gel—not liquid, but with some “give.” They are classified as non–form-stable. Still under evaluation by the FDA are fifth-generation, form-stable, more highly cohesive, silicone gel–filled implants, with a consistency similar to thick gelatin, soft brie cheese, or gummy candies (Figure 1 and box on this page). These implants have shown improved outcomes in aesthetics, reduced capsular contracture, and longevity, and likely will foreshadow future developments and improvements within the industry.
See Table 1 at the bottom for a history of silicone gel–filled breast implants.
A Precise Technique
Implant selection and technique are based on measurable dimensions and soft-tissue characteristics determined during the consultation. These can be separated into individual steps.
My primary goal in patient consultation is to first define the patient’s desires. This is of paramount importance in any discussion about breast implants. Every woman has a preconceived notion of what size and shape breasts she really wants. This desire must be weighed against the options her tissues offer; in some cases, there is a vast disconnect between what she wants and what can reasonably and safely be achieved.
The implant selection techniques, preoperative planning, and surgical technique that have evolved in my practice have the primary goal of enhancing outcomes and therefore patient satisfaction. I call this process the “biodimensional approach” (Figure 2).
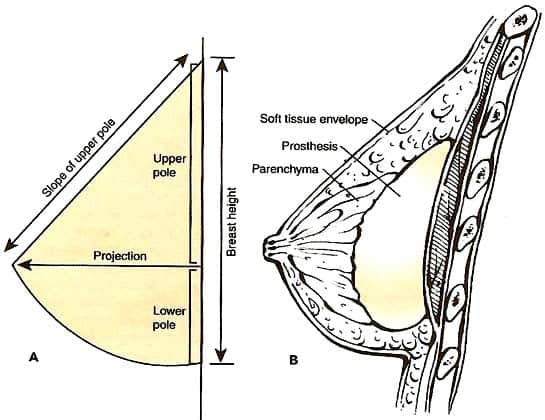 |
| Figure 2. The aesthetic breast form can be enhanced by the proper selection and use of silicone gel breast implants through a “biodimensional approach.” 2 |
The next step is to examine the amount and location of the patient’s glandular tissue, as well as the shape and structure of her chest wall and the surrounding tissues. On the basis of this evaluation and these precise measurements and calculations, I select the most appropriate width or base of the implant, which generally should not exceed the width of her breast tissue.
For example, a woman with a 32-inch chest may require a slightly narrower implant than a woman with a 36-inch chest. More specifically, a breast width of 12.5 cm would accommodate an implant 12.0 cm or less in width. The next calculation is the height of the implant—the height needed to create a natural-looking breast shape. (Of course, round implants would be the same height and width.)
Last is the determination of the preferred implant projection. The site, or pocket, into which the implants are placed is based on several factors, but primarily on the amount and quality of the soft tissue that will cover the implant. Incision options are also individualized, and the pros and cons of each must be understood. For an example of the process, see Figure 3.
The entire process—from good physician–patient communication through surgical planning and execution—must be virtually flawless so that the total clinical experience for the patient is positive. It is imperative to involve the patient in this process from start to finish so that you work as a team to make choices that will produce the best outcome possible.
This biodimensional method of implant selection and sizing, along with the surgical approach for inserting and positioning breast implants, while based on previous outcome experience, may need to be adjusted to accommodate the patient’s individualized anatomy. The shape and size of the breasts prior to surgery will influence the recommended treatment and the final results. If the breasts are not the same size or shape before surgery, it is unlikely that they will be completely symmetrical afterward, even when intraoperative adjustments are made.
Potential Risks
Whereas improved devices and techniques have lessened the risks associated with breast augmentation, FDA clinical trial augmentation data show that there is room for improvement. The reoperation rate for primary breast augmentation (using fourth-generation silicone gel implants) is approximately 20% at 4 years. The main reason for this is capsular contracture: 13% at 4 years, followed by patient desire for size change.
Other complications reported are breast pain, swelling, hematoma, implant malposition, rippling or edge visibility of implants, asymmetry, and implant rupture. We surgeons have the responsibility to lessen these statistics in our own practices.
A breakthrough in reducing complications is seen with the 3-year form-stable (fifth-generation) FDA data: A little more than 1% of women developed capsular contracture, and the reoperation rate was half that of fourth-generation devices. Likewise, MRI data from Europe show that non–form-stable implants have a rupture rate of 8% at 10 years, while the highly cohesive, form-stable implants have a 0.3% rupture rate at 6 to 8 years.
All physicians must be certified by manufacturers to use silicone gel breast implants. The “directions for use” document and the patient label approved by the FDA must be reviewed by the physician and patient, respectively. All adverse effects and complications documented during the clinical trials are contained in these documents and must be well understood by all women who are considering silicone gel augmentation.
To continue gathering data on the long-term outcomes of silicone gel breast implants, the FDA requires all women who receive implants in the United States to be a part of a 10-year postsurgical follow-up evaluation program.
 |
| See also “Back in Business” by Malcolm D. Paul, MS, FACS, in the April 2007 issue of PSP. |
Patients who request an outcome that produces a disproportionately large breast size must consider that such a choice can place them at risk for a suboptimal long-term outcome and the subsequent need for reoperation. The placement of oversized breast implants that exceed the normal dimensions of the breast can produce irreversible tissue thinning, implant dropout, and visible or palpable rippling and synmastia, a condition in which breast mounds are abnormally located in the center of the chest. Large-volume primary augmentation or revision with larger implants (greater than 400 mL) may increase the risk of complications and lead to reoperations.
Today, with the new silicone gel, as well as the highly cohesive form-stable silicone gel implants, American women have fresh choices and options to actualize their goals.
Table 1. Evolution of Silicone Gel-Filled Breast Implants2
-
First Generation (1962-1970)
-
Thick, two-piece shell
Smooth surface with Dacron fixation patches
Anatomically shaped (teardrop)
Viscous silicone gel -
Second Generation (1970-1982)
-
Thin, slightly permeable shell
Smooth surface (no Dacron patches)
Less viscous silicone gel -
Third Generation (1982-1992)
-
Thick, strong, low-bleed shell
Smooth surface
Round shape
More viscous silicone gel -
Fourth Generation (1993-present)
-
Thick, strong, low-bleed shell*
Smooth and textured surfaces
Round and anatomically shaped
More viscous (cohesive) silicone gel* -
Fifth Generation (1993-present)
-
Thick, strong, low-bleed shell*
Smooth and textured surfaces
Round and diverse anatomical shapes
Enhnaced cohesive and form-stable silicone gel
* In accordance with technical parameters established by the ASTM.
G. Patrick Maxwell, MD, FACS, is a board-certified plastic surgeon in private practice in Nashville, Tenn, and is an associate clinical professor in the department of plastic surgery at Vanderbilt University School of Medicine in Nashville. His first book, Beautiful Breasts, will be published this fall by MDPublish. He can be reached via his Web site, www.maxwellaesthetics.com.
References
- Hedén P, Boné B, Murphy DK, Slicton A, Walker PS. Style 410 cohesive silicone breast implants: Safety and effectiveness at 5 to 9 years after implantation. Plast Reconstr Surg. 2006;118:1281–1287.
- Maxwell GP, Baker MB. In: Spear SL, ed. Surgery of the Breast. 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2006:1237–1260.