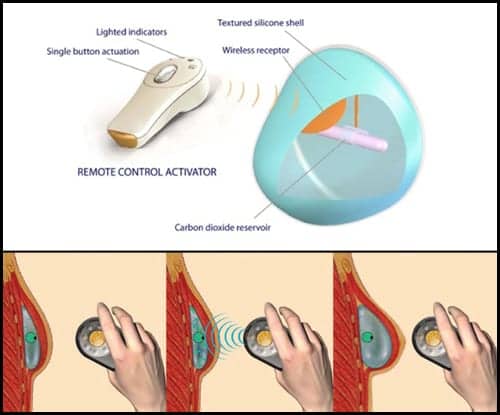
AirXpanders Inc has received US Food and Drug Administration (FDA) 510(k) clearance to market a smooth shell version of the AeroForm Tissue Expander, the company announces.
“We are very pleased to receive FDA 510(k) clearance for the smooth shell AeroForm tissue expander,” says Frank Grillo, president and CEO of AirXpanders, in a media release.
“The plastic surgery community has embraced the textured version of our tissue expander, and they have also been asking us to provide a smooth, untextured version of AeroForm. This clearance enables market release in the US, and we have already submitted our regulatory application for CE mark approval.”
[Source(s): AirXPanders Inc, PR Newswire]