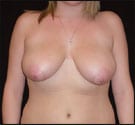
Apyx Medical Corp., developer of Helium Plasma Technology, marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market, announces it has submitted a 510(k) premarket notification to the U.S. FDA. The 510(k) submission is intended to expand its general indication to include a specific indication for the use of the Renuvion APR Handpiece in subcutaneous dermatological and aesthetic procedures to achieve thermal coagulation/contraction to improve the appearance of lax (loose) skin in the neck and submental region.
Charlie Goodwin, CEO and president of Apyx Medical Corp., addressed the premarket submission, commenting: “We are excited to announce the submission of this new request for 510(k) clearance ahead of our prior expectations, which is intended to enable Apyx Medical to market and sell our Renuvion APR Handpiece for use to improve the appearance of lax (loose) skin in the neck and submental region.”
What’s more, he says, “This 510(k) submission represents a key milestone in our long-term strategy to pursue specific clinical indications related to our target procedures in the cosmetic or aesthetic surgery market. The 510(k) is primarily supported by safety and effectiveness data from our U.S. IDE clinical study evaluating the use of Renuvion technology in the neck and submental region to improve the appearance of lax (loose) skin and reflects a multi-year effort from our clinical and regulatory teams.”
Goodwin adds that receiving FDA clearance would allow the company to “expand the addressable market opportunity for our Renuvion technology by enabling us to market to surgeons and patients for this specific indication.”