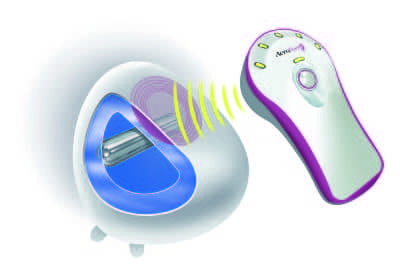
A novel method—the AirXpanders AeroForm tissue expander—may prove to be a viable alternative for some women pursuing breast reconstruction. So far, four randomized controlled clinical trials—the Australian Patient Activated Controlled Expansion (PACE) I and II, the AeroForm Patient Controlled Tissue Expansion for Breast Reconstruction (ASPIRE), and the ongoing US-based AirXpanders Patient Activated Controlled Tissue Expander System for Breast Reconstruction (XPAND)—have all yielded consistent and positive results in terms of days of expansion, completion of expansion, and exchange to a permanent implant.
The device is already approved in Australia, and the company plans to file a 510(k) application with the US Food and Drug Administration by the end of 2014 or early 2015.
AeroForm holds numerous advantages compared with traditional saline tissue expanders, explains ASPIRE and PACE study author and Australian plastic surgeon Tony Connell, MD, FRACS. “First of all, I do not have to inject the patient with a needle to fill the device,” he says. “Although the breast tissue has been removed during the mastectomy, no one likes the idea of having an injection unless necessary.”
Secondly, he says, expansion occurs much faster than with the standard saline expander. “With the AirXpander, I can have the submuscular pocket fully expanded in 19 days versus several months with the saline devices.”
The Shape of Things to Come
In addition, the patient can expand from home, which means less disruption in routine. Taken together, these benefits enhance quality of life—especially for women who are eager to put their breast cancer behind them and get on with the rest of their lives.
“The remote control system provides convenience for both physician and patients—no needles, no setting up a sterile field, no painful bolus of saline, and fewer visits to the office,” adds Kamakshi R. Zeidler, MD, an aesthetic and reconstructive plastic surgeon in the Los Gatos, San Jose, and greater South Bay Area of California who is also the lead author of the XPAND trial. “This means that the whole process of reconstruction can be completed faster.”
Another perk is the shape of the AirXpander, Zeidler says. “The expander is designed to create a very nice shape with good projection in the bottom part of the breast, which is where it is most needed to create an aesthetically shaped breast.”
She predicts that the device, if approved, will be especially attractive for patients who have to travel great distances to see their plastic surgeons, as well as those with early-stage disease and those who undergo prophylactic mastectomies for breast cancer gene mutations. The latter two groups do not require adjuvant therapies and are therefore eager to move through the reconstructive process quickly, she says. “If the early results of the trial continue at the same rate, this expander can likely get them to their end result in about a third of the time.”
Unanswered Questions
Complications associated with the AeroForm device typically mirror many of those seen with saline expansion, and largely arise from wound healing issues where the flaps are devascularized or do not heal properly. Until recently, however, there were some lingering questions about how the new technology meshes with radiotherapy and/or air travel. Now, new research from ASPIRE slated for presentation at the American Society of Plastic Surgeons meeting in Chicago suggests that both are safe for AirXpander patients.
“The device has shown that it can withstand a cumulative dose of over 75 Gy (well beyond the amount delivered during typical radiotherapy regimens for patients who have had mastectomy), and a recently published study in Practical Radiation Oncology supports that successful radiotherapy can be delivered to the chest wall and surrounding area even with the AeroForm device in place,” Connell says.
Patients were grounded from air travel during the early days of the trials due to concern about how—or if—altitude would affect the device, but this restriction has since been relaxed. “Because the device is filled with a gas, there is some expansion that takes place as the device goes up in altitude. Because all commercial airlines have pressurized cabins, the amount of expansion is limited,” he says. So far, 15 AirXpander patients have flown—all without incident.
“What we have learned in terms of a ‘best practice’ is to tell the patient who intends to fly to discontinue dosing 1 to 2 weeks before her trip, leave her dose controllers at home, and then resume dosing when she returns,” he says. “Since the AeroForm allows for such rapid expansion, this has become a nonissue in clinical practice.”
Denise Mann is the editor of Plastic Surgery Practice. She can be reached at [email protected].