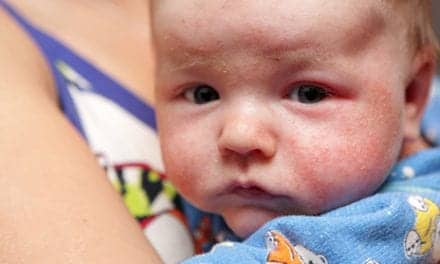
This week during the annual meeting of the American Academy of Dermatology in San Diego, Robert Sidbury, M.D., addressed developments in atopic dermatitis treatments since the publication of treatment guidelines in 2014.
The U.S. Food and Drug Administration approved the novel topical PDE-4 inhibitor crisaborole for mild-to-moderate atopic dermatitis in December 2016, and the IL4/13 inhibitor dupilumab for moderate-to-severe disease roughly 3 months later, in March 2017.
Click here to view original web page at dermatologytimes.modernmedicine.com