New research shows that KYTHERA Biopharmaceuticals Inc ATX-101 performed well in two Phase III trials.
ATX-101 is a purified synthetic version of deoxycholic acid, a naturally occurring molecule in the body that aids in the breakdown of fat. It is being investigated as a treatment for submental fat. Currently, there is no injectable drug approved by the US Food and Drug Administration (FDA) to reduce submental fat.
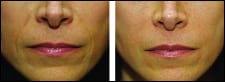
The new data demonstrate that the majority of ATX-101 subjects reported satisfaction with their treatment as well as improvements on visual and psychological impacts of their “chin fat.” Additionally, the vast majority of subjects had a reduction in submental fat as assessed independently by clinicians and patients. Most adverse events were transient, local to the treatment area, and mild to moderate in severity.
Top line data from the REFINE-1 and REFINE-2 studies were presented at the American Society for Dermatologic Surgery’s annual meeting in Chicago.
Stay tuned for an indepth look at ATX-101 in November issue of Plastic Surgery Practice.