An estimated 12% or more of women will be diagnosed with breast cancer in their lifetime. In 2007, this translated into more than 1.3 million new diagnoses of invasive breast cancer worldwide, with close to 200,000 occurring in the United States alone.
Surgical therapy (mastectomy or partial mastectomy) remains an integral part of the treatment for breast cancer. With an understanding of the psychological benefits of breast reconstruction, the plastic surgeon has assumed an integral role in the multidisciplinary treatment of women with breast cancer.
Armed with an expanded anatomic knowledge and refined surgical techniques, the modern practitioner can also offer elegant and aesthetic options for autologous breast reconstruction.
PARTIAL MASTECTOMY RECONSTRUCTION AND ONCOPLASTIC BREAST SURGERY
Breast conservation (partial mastectomy and breast irradiation) remains the preferred locoregional treatment for many women with early-stage breast cancer. In preserving a large part of the native breast, breast conservation theoretically avoids the psychological burden of mastectomy. However, studies have shown that 20% to 30% of women undergoing breast-conservation therapy are dissatisfied with the aesthetic outcome. Asymmetry of the nipple-areolar complex, as well as discrepancies in volume and shape, are undesired but common outcomes for many women who have undergone breast-conservation therapy. Secondary correction of these deformities following radiation therapy is often unpredictable and carries complication rates approaching 50%.
In order to address the untoward results of breast conservation, plastic surgeons developed oncoplastic breast reconstructive techniques. These techniques utilize reduction mammoplasty and mastopexy principles to reshape the breast following partial mastectomy.
In addition, regional tissues such as the latissimus dorsi musculocutaneous flap or the muscle-sparing thoracodorsal artery perforator (TAP) flap can be utilized to recruit larger volumes of nonradiated tissue when necessary. These techniques can be incorporated at the same operative setting as partial mastectomy (immediate). This relies on frozen section analysis to confirm negative margins. Alternatively, reconstruction can be deferred until a second operative setting, following final pathologic assessment of margin status (delayed immediate). In either case, breast reshaping precedes the onset of breast irradiation.
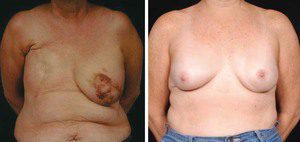
Figure 1. Modern breast surgery has evolved from the traditional radical mastectomy (left) to lesser-invasive techniques such as the nipple-sparing mastectomy, pictured here after reconstruction with an abdominal free tissue flap.
Oncoplastic reconstruction has been shown to have a positive impact on quality of life and self-esteem when compared with traditional breast-conservation therapy. In general, traditional breast conservation can be performed satisfactorily in situations where less than 10% of the native breast volume is excised. However, when more than 15% to 20% is excised, oncoplastic techniques may be helpful in preserving the natural appearance of the breast.
For woman with larger breasts, oncoplastic techniques can permit excision of up to 1,000 grams of breast tissue while maintaining an aesthetic breast contour. With the advent of these techniques, women who would traditionally require mastectomy based on the size and location of their tumor may now be candidates for breast preservation.
Although large, long-term studies on oncoplastic reconstruction are not yet available, preliminary data suggests that survival and recurrence rates are similar to those of traditional breast conservation.
Not all patients are candidates for oncoplastic surgery, and a decision that considers each patient’s tumor characteristics and overall risk should be made by a multidisciplinary breast cancer treatment team.
MASTECTOMY RECONSTRUCTION AND THE EVOLUTION OF TECHNIQUE
Historically, oncologic safety and aesthetic outcomes have been competing interests in breast reconstruction. However, advances in both oncologic and reconstructive breast surgery have heralded a new era in which oncologic safety and aesthetic outcome are no longer mutually exclusive.
A major advance in oncologic breast surgery has been the development of skin-sparing techniques, including skin- and nipple-sparing mastectomy.
From a reconstructive standpoint, skin-sparing mastectomy is advantageous in that it requires shorter scars and preserves the native skin envelope, including the inframammary fold, which can be difficult to recreate. Skin-sparing mastectomy is indicated in early-stage breast cancers and offers local recurrence rates similar to traditional mastectomy.
Realizing the psychological impact of nipple loss and the shortcomings of nipple-reconstruction techniques, surgeons extended the concept of skin-sparing mastectomy to nipple-sparing mastectomy.
In addition to retaining the native skin envelope, the nipple-sparing mastectomy also preserves the areola as well as the dermis and epidermis of the nipple while the underlying ducts are removed.
The nipple-sparing mastectomy may be an option in select patients, such as those undergoing prophylactic mastectomy for risk reduction. The risk of a retained occult malignancy is diminished by intraoperative frozen-section evaluation of the subareolar tissue.
Concurrent with the evolution of mastectomy techniques, reconstructive surgery has seen important advances in anatomic knowledge and refinements in surgical technique.
A better understanding of regional perforator anatomy has led to the development of perforator-based soft tissue flaps, which spare muscle function and avoid much of the donor-site morbidity inherent in musculocutaneous flaps.
The most important advance in plastic surgery technique is the ability for free tissue transfer utilizing microsurgical techniques. This allows breast reconstruction using the patient’s own tissue and recreates a breast that is similar in look and feel to the patient’s native breast. Refinements in microsurgical technique allow routine use of free tissue transfer in a safe and reproducible fashion. Currently, noninvasive preoperative perforator imaging is becoming an important adjunct to delineate an individual patient’s anatomy and safely expedite the surgical procedure.
IMPLANT-BASED RECONSTRUCTION
Although the focus of this article is autologous breast reconstruction, implant-based reconstruction remains a popular choice for many patients and one that can also achieve excellent aesthetic results. Many women, however, prefer to undergo reconstruction using their own tissue and avoid implantation of any device that is considered “foreign.”
Despite a lack of credible scientific evidence linking silicone implants to a whole host of medical ailments, many women still adhere to the negative media attention surrounding silicone implants in the late 1990s and opt out of implant-based reconstruction.
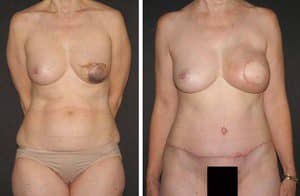
Figure 2. Improvement in abdominal contour with abdominal soft tissue flaps. Preoperative (left) and postoperative (right) photos of a patient who has undergone breast reconstruction with an abdominal soft tissue flap.
Similarly, not all patients are necessarily good candidates for implant-based reconstruction. The most common factor that confounds implant-based reconstruction remains a patient’s past history of—or future need for—radiation therapy. Because the complication rates of implant-based reconstruction in an irradiated field are higher than in non-radiated tissue (that is, increased rates of capsular contracture), many surgeons recommend autologous reconstruction in this situation.
FREE TISSUE TRANSFER
Anatomic knowledge and surgical technique have evolved to the point that free tissue transfer can now be performed safely and reliably in most modern plastic surgical units. Autologous reconstruction is an appealing option to many patients who wish to avoid implantation of prosthetic material. Several breast reconstruction options utilize free tissue transfer.
The abdominal donor site has remained the workhorse for autologous breast reconstruction since the introduction of the free transverse rectus abdominis myocutaneous (TRAM) flap by Hartrampf et al in 1982.
From the patient’s perspective, the abdominal donor site is advantageous in that it removes skin and fat from the lower abdomen in a manner analogous to abdominoplasty. For the breast cancer patient who is often middle-aged and has completed child-bearing, an improvement in abdominal contour represents an appealing aspect of this procedure.
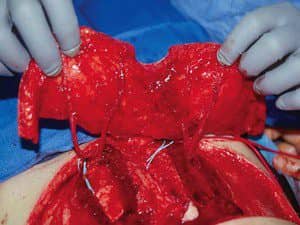
Figure 3. Deep inferior epigastric artery perforator flap. The rectus abdominis muscles and abdominal wall fascia have been preserved.
For the surgeon, abdominal flaps typically provide enough soft tissue bulk to successfully recreate the breast mound and can be harvested simultaneously with mastectomy without the need for repositioning.
The free TRAM traditionally involved harvest of the entire rectus abdominis muscle and overlying fascia, adipose tissue, and skin based on the deep inferior epigastric artery, the primary blood supply to this tissue. The abdominal wall morbidity associated with the harvest of the entire rectus muscle and fascia, particularly in cases of bilateral harvest, is a limitation of the free TRAM that has stimulated plastic surgeons to develop less-invasive techniques. These techniques include the muscle-sparing free TRAM (MS TRAM), deep inferior epigastric artery perforator (DIEP) flap, and superficial inferior epigastric artery (SIEA) flap.
The MS TRAM involves harvesting a small cuff of rectus abdominis muscle, leaving behind innervated, functional rectus muscle and overlying fascia. One step further, the DIEP and SIEA flaps preserve the entire rectus muscle and overlying fascia.
The DIEP flap relies on perforators from the deep inferior epigastric artery, which penetrate the rectus muscle and overlying fascia, and supply the overlying skin and subcutaneous fat. In harvesting the DIEP flap, the rectus muscle and fascia are split to facilitate dissection of these perforators. Because the fascia and muscle are left in their entirety, abdominal wall morbidity is minimized.
In a similar fashion, the SIEA flap leaves the rectus muscle and fascia undisturbed altogether. This flap, based on the superficial inferior epigastric artery and vein, is raised above the fascia of the anterior abdominal wall—avoiding abdominal wall morbidity. The superficial inferior epigastric artery can be diminutive in many patients. It is estimated that less than one-third of patients have vascular anatomy that allows transfer of this flap.
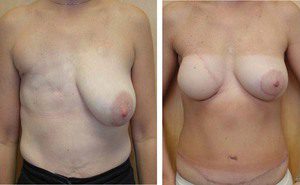
Figure 4. Deep inferior epigastric artery perforator flap. Pre-operative (left) and postoperative photos of a patient who has undergone delayed breast reconstruction with a DIEP flap (right).
These less-invasive alternatives to the traditional TRAM flap provide benefits in terms of abdominal wall morbidity but require tedious and time-consuming intramuscular dissection. In an effort to improve the efficiency of flap harvest, a new trend employs preoperative imaging to delineate the vascular anatomy of the DIEP and SIEA systems. Most commonly, surgeons have turned to preoperative CT Angiography to plot the location of the deep inferior epigastric artery perforators. Armed with this information, the surgeon can target the areas of interest, safely expediting the operation. In addition, preoperative imaging can verify the patency of the deep and superficial inferior epigastric vessels in women who have had previous gynecologic surgery through a lower abdominal incision. Although preoperative imaging is a relatively new trend, it holds promise in reducing operative times in a safe manner.
OTHER DONOR SITES
Although the lower abdomen has become the workhorse donor site, not every patient is a candidate for a free flap from this area. For example, slender women with a paucity of lower abdominal adiposity may not have enough volume for an aesthetic reconstruction, particularly when a bilateral reconstruction is planned. Similarly, a history of previous open abdominal surgery may preclude the abdomen as a donor site.
Likewise, any operation that has compromised the integrity of the perforating vessels (abdominoplasty) precludes lower abdominal flaps. Previous laparoscopic surgery (such as laparoscopic cholecystectomy) or gynecologic surgery via a Pfannenstiel incision is not typically contraindications to lower abdominal free flaps. For women who are not candidates for the lower abdominal donor site, other reliable options for autologous reconstruction exist.
The medial thigh serves as an excellent alternative for women who have a natural deposition of fat in this region. Based on vessels from the medial femoral circumflex system, the transverse upper gracilis flap captures the soft tissue from the upper, inner thigh as well as the underlying gracilis muscle—an expendable muscle residing in the medial thigh.
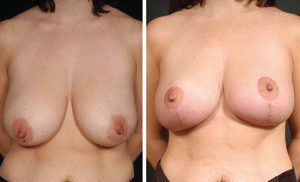
Figure 5. Oncoplastic surgery. This patient with macromastia and breast cancer underwent partial mastectomy utilizing an inferior pedicle Wise pattern technique.
Advantages of this flap include minimal donor-site morbidity, a concealed scar, and constant anatomy. Furthermore, the semicircular shape of the flap permits final shaping of the flap in a conical fashion, similar to that of the native breast. As with abdominal flaps, the adipose tissue of the inner thigh is soft and resembles the texture of the native breast.
The gluteal region also serves as an additional alternative to abdominal-based reconstruction. Based on the perforators of the superior gluteal artery, the superior gluteal artery perforator flap includes tissue from the upper buttock region, leaving an inconspicuous scar at the junction of aesthetic units. The firm consistency of the adipose tissue in this region and the need for intraoperative repositioning have prevented widespread acceptance as a first-line option for microsurgical breast reconstruction. Nonetheless, it represents a valuable alternative in the armamentarium of the reconstructive microsurgeon.
POSTMASTECTOMY LYMPHEDEMA
Upper-extremity lymphedema is estimated to occur in up to 40% of breast cancer patients and represents one of the most debilitating sequelae of mastectomy. Most frequent in women who have undergone axillary dissection and radiation therapy, lymphedema is a chronic, debilitating condition that results in unremitting upper-extremity swelling, deformity, and repeated bouts of infection.
Noninvasive therapies, including external compression, manual lymphatic drainage, exercise, and skin care have been the primary treatment of lymphedema. However, these interventions are often time-consuming and painful, and long-term compliance is problematic. Surgical therapy has traditional been reserved for patients who fail to respond to conservative measures. Excisional procedures have included surgical debulking, as well as suction-assisted removal of lymphedematous tissue. Surgical debulking is typically reserve for the most advanced cases, where the size of the affected extremity affects activities of daily living.
These invasive interventions often yield suboptimal aesthetic results and carry a significant morbidity. Less invasive debulking via suction-assisted techniques are plagued by technical difficulty due to fibrosis of the skin and subcutaneous tissue, as well as significant recurrence rates.
The limitations of excisional procedures have led to the development of physiologic microsurgical procedures for the treatment of lymphedema. These include lymphaticovenous anastomoses and vascularized lymph node transfers. Lymphaticovenous procedures employ supermicrosurgical techniques to anastomose subdermal lymphatics to adjacent venules.
Vascularized lymph node transfer represents an exciting development in the treatment of postmastectomy lymphedema. This technique imports healthy lymph nodes from a distant region of the body. Most commonly, a skin paddle from the upper leg with its associated underlying superficial groin lymph nodes are transferred based on the superficial circumflex iliac artery and vein. Utilizing microsurgical techniques, the vessels are anastomosed to the superficial branch of the radial artery and a branch of the cephalic vein. Alternatively, anastomoses to the circumflex scapular vessels can be performed.
From an aesthetic perspective, this technique yields favorable results, as the skin paddle can be de-epithelialized and buried at a second stage, leaving a straight-line scar.
CONCLUSION
A wide variety of autologous reconstructive options are currently available for the breast cancer patient. Muscle-sparing techniques for mastectomy reconstruction (MS TRAM, DIEP, and SIEA) provide aesthetic reconstructive options while minimizing abdominal donor-site morbidity. Oncoplastic breast surgery is a valuable option to avoid deformity that can result from breast-conservation therapy.
Lucio A Pavone, MD; Iris A. Seitz, MD, PhD; and Loren S. Schechter, MD, FACS, are plastic surgeons at University Plastic Surgery, SC in Morton Grove, Ill.



